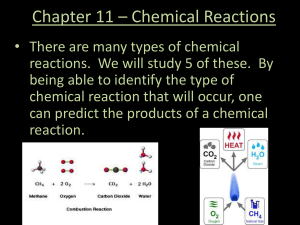
unit iv – stoichiometry 1
... VIII. Laws of Chemical Combination A) Law of Definite Proportions – different samples of the same compound will always contain the same proportion of elements by mass B) Law of Multiple Proportions – if two elements can combine to form more than one compound, the masses of one element that combine w ...
... VIII. Laws of Chemical Combination A) Law of Definite Proportions – different samples of the same compound will always contain the same proportion of elements by mass B) Law of Multiple Proportions – if two elements can combine to form more than one compound, the masses of one element that combine w ...
AP® Chemistry
... morning or afternoon is available to administer this practice exam. If a schedule does not permit one time period for the entire practice exam administration, it would be acceptable to administer Section I one day and Section II on a subsequent day. Many students wonder whether or not to guess the a ...
... morning or afternoon is available to administer this practice exam. If a schedule does not permit one time period for the entire practice exam administration, it would be acceptable to administer Section I one day and Section II on a subsequent day. Many students wonder whether or not to guess the a ...
Ch3
... Solve the following conversions How many atoms of silver are in 3.50 moles of silver? Determine the number of moles of carbon disulfide in 34.75 grams of CS2. Determine the number of sulfur atoms in 34.75 grams of CS2. Copyright McGraw-Hill 2009 ...
... Solve the following conversions How many atoms of silver are in 3.50 moles of silver? Determine the number of moles of carbon disulfide in 34.75 grams of CS2. Determine the number of sulfur atoms in 34.75 grams of CS2. Copyright McGraw-Hill 2009 ...
This article was published in an Elsevier journal. The attached copy
... scrubbing Depending on the feedstock of H2 S-containing gas for Section 1, the SO2 for the Bunsen reaction may be in a mixture with CO2 or CH4 and other low hydrocarbons. Whether this SO2 -containing mixture can be directly used for the Bunsen reaction is determined by whether there are reactions be ...
... scrubbing Depending on the feedstock of H2 S-containing gas for Section 1, the SO2 for the Bunsen reaction may be in a mixture with CO2 or CH4 and other low hydrocarbons. Whether this SO2 -containing mixture can be directly used for the Bunsen reaction is determined by whether there are reactions be ...
wiley_ch6_Chemical_Equilibrium
... Almost all systems come to equilibrium Where equilibrium lies depends on system Some systems’ equilibrium hard to detect Essentially no reactants or no products present Jespersen/Brady/Hyslop ...
... Almost all systems come to equilibrium Where equilibrium lies depends on system Some systems’ equilibrium hard to detect Essentially no reactants or no products present Jespersen/Brady/Hyslop ...
Chemistry Skills Practice Assignments
... 2. For which substance, A or B, does the freezing point decrease as the pressure is increased? 3. One of the substances behaves more like most other substances. Which substance and what property allows you to tell? 4. Assuming that the temperature scales for both phase diagrams are the same, which c ...
... 2. For which substance, A or B, does the freezing point decrease as the pressure is increased? 3. One of the substances behaves more like most other substances. Which substance and what property allows you to tell? 4. Assuming that the temperature scales for both phase diagrams are the same, which c ...
Pirogov National Medical Univercity of Vinnitsa
... Early beginning of each semester the teacher must instruct a group of students on policy compliance rules work in the chemical laboratory and safety equipment. Students must confirm the knowledge of safety rules in their own signatures in a magazine. Accidents (burns, wounds, poisoning) in the labor ...
... Early beginning of each semester the teacher must instruct a group of students on policy compliance rules work in the chemical laboratory and safety equipment. Students must confirm the knowledge of safety rules in their own signatures in a magazine. Accidents (burns, wounds, poisoning) in the labor ...
2 - Scheikundeolympiade
... If yes, calculate the concentration. Yes (1 pt) We can suppose that this solution would be quite acidic, so the 3rd and 4th dissociation steps can be disregarded. (1 pt) The following equations are thus ...
... If yes, calculate the concentration. Yes (1 pt) We can suppose that this solution would be quite acidic, so the 3rd and 4th dissociation steps can be disregarded. (1 pt) The following equations are thus ...
Principles of Chemistry 1 and 2 Notes
... (treat single, double and triple bonds as the same). 3 sets (no lone pair) ----> geometry is trigonal planar. ------------------------------------------------------b. BeF2 (beryllium is underlined as a central atom) Lewis structure is as follows: :::F - Be - F::: There are two single bonds of beryll ...
... (treat single, double and triple bonds as the same). 3 sets (no lone pair) ----> geometry is trigonal planar. ------------------------------------------------------b. BeF2 (beryllium is underlined as a central atom) Lewis structure is as follows: :::F - Be - F::: There are two single bonds of beryll ...
chapter 4 - reactions in solution
... For example, compounds such as NaCl and KCl are very soluble in water, but AgCl is only very slightly soluble. The differences in the solubility of ionic compounds in water depend on the relative attractions of the ions for each other and the interactions of the ions for water molecules. If ion- ...
... For example, compounds such as NaCl and KCl are very soluble in water, but AgCl is only very slightly soluble. The differences in the solubility of ionic compounds in water depend on the relative attractions of the ions for each other and the interactions of the ions for water molecules. If ion- ...
Moles Class Packet Unit 2
... 8. If 3.00 moles of Iron (III) oxide are used, how many moles of Iron are formed? 9. If 2.50 moles of CO are used, how many moles of carbon dioxide are formed? 10. If 8.56 moles of iron were produced, how many moles of the iron ore were used? 11. If 25.68 grams of iron (III) oxide were used, how man ...
... 8. If 3.00 moles of Iron (III) oxide are used, how many moles of Iron are formed? 9. If 2.50 moles of CO are used, how many moles of carbon dioxide are formed? 10. If 8.56 moles of iron were produced, how many moles of the iron ore were used? 11. If 25.68 grams of iron (III) oxide were used, how man ...