* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
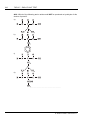
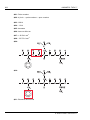
Download CfE Higher Chemistry Unit 3: Chemistry in Society
Chemical potential wikipedia , lookup
Host–guest chemistry wikipedia , lookup
Van der Waals equation wikipedia , lookup
Thermodynamics wikipedia , lookup
Heat transfer physics wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Reaction progress kinetic analysis wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Work (thermodynamics) wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Acid–base reaction wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Marcus theory wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Atomic theory wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Enzyme catalysis wikipedia , lookup
Rate equation wikipedia , lookup
Equation of state wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
George S. Hammond wikipedia , lookup
Electrochemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
SCHOLAR Study Guide CfE Higher Chemistry Unit 3: Chemistry in Society Authored by: Emma Maclean (George-Heriot’s School) Reviewed by: Diane Oldershaw (Menzieshill High School) Previously authored by: Peter Johnson Brian T McKerchar Arthur A Sandison Heriot-Watt University Edinburgh EH14 4AS, United Kingdom. First published 2014 by Heriot-Watt University. This edition published in 2016 by Heriot-Watt University SCHOLAR. Copyright © 2016 SCHOLAR Forum. Members of the SCHOLAR Forum may reproduce this publication in whole or in part for educational purposes within their establishment providing that no profit accrues at any stage, Any other use of the materials is governed by the general copyright statement that follows. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, without written permission from the publisher. Heriot-Watt University accepts no responsibility or liability whatsoever with regard to the information contained in this study guide. Distributed by the SCHOLAR Forum. SCHOLAR Study Guide Unit 3: CfE Higher Chemistry 1. CfE Higher Chemistry Course Code: C713 76 ISBN 978-1-909633-22-3 Print Production and Fulfilment in UK by Print Trail www.printtrail.com Acknowledgements Thanks are due to the members of Heriot-Watt University's SCHOLAR team who planned and created these materials, and to the many colleagues who reviewed the content. We would like to acknowledge the assistance of the education authorities, colleges, teachers and students who contributed to the SCHOLAR programme and who evaluated these materials. Grateful acknowledgement is made for permission to use the following material in the SCHOLAR programme: The Scottish Qualifications Authority for permission to use Past Papers assessments. The Scottish Government for financial support. The content of this Study Guide is aligned to the Scottish Qualifications Authority (SQA) curriculum. All brand names, product names, logos and related devices are used for identification purposes only and are trademarks, registered trademarks or service marks of their respective holders. i Contents 1 Getting the most from reactants 1.1 Prior knowledge . . . . . . . . . . . . . . . . 1.2 Introduction . . . . . . . . . . . . . . . . . . 1.3 The design of industrial chemical processes 1.4 Mole calculations . . . . . . . . . . . . . . . 1.5 Molar volume . . . . . . . . . . . . . . . . . 1.6 Reacting volumes . . . . . . . . . . . . . . . 1.7 Percentage yields . . . . . . . . . . . . . . . 1.8 The atom economy of a process . . . . . . . 1.9 Excess . . . . . . . . . . . . . . . . . . . . . 1.10 Summary . . . . . . . . . . . . . . . . . . . . 1.11 Resources . . . . . . . . . . . . . . . . . . . 1.12 End of topic test . . . . . . . . . . . . . . . . 2 Chemical equilibria 2.1 Prior knowledge . . . . . . . . . 2.2 Reversible reactions . . . . . . . 2.3 Dynamic equilibrium . . . . . . 2.4 Altering the equilibrium position 2.5 The Haber process . . . . . . . 2.6 Summary . . . . . . . . . . . . . 2.7 Resources . . . . . . . . . . . . 2.8 End of topic test . . . . . . . . . 3 Chemical energy 3.1 Prior knowledge . . . . . . 3.2 Introduction . . . . . . . . 3.3 Potential energy diagrams 3.4 Enthalpy changes . . . . . 3.5 Introduction to Hess's law 3.6 Bond enthalpies . . . . . . 3.7 Summary . . . . . . . . . . 3.8 Resources . . . . . . . . . 3.9 End of topic test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 4 4 5 10 22 28 32 37 43 46 47 48 . . . . . . . . 53 55 56 57 61 67 72 73 74 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 79 83 84 86 95 106 116 118 120 121 4 Oxidising or reducing agents 4.1 Prior knowledge . . . . . . . . . . . . . . . . . . . . . . . . . 4.2 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.3 Elements as oxidising and reducing agents . . . . . . . . . . 4.4 Molecules and group ions as oxidising and reducing agents . . . . . . . . . . . . . . . . . . . . . . . . 129 132 133 134 139 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ii CONTENTS 4.5 4.6 4.7 4.8 4.9 4.10 4.11 4.12 4.13 Uses for strong oxidising agents . Ion-electron half equations . . . . Combining ion-electron equations Complex ion-electron equations . Redox titrations . . . . . . . . . . Summary cloze test . . . . . . . . Summary . . . . . . . . . . . . . . Resources . . . . . . . . . . . . . End of topic test . . . . . . . . . . 5 Chromatography 5.1 Prior knowledge . . . . . . . . . 5.2 Introduction . . . . . . . . . . . 5.3 Paper chromatography . . . . . 5.4 Gas-liquid chromatography . . . 5.5 Size-exclusion chromatography 5.6 Summary . . . . . . . . . . . . . 5.7 Resources . . . . . . . . . . . . 5.8 End of topic test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 142 145 148 150 155 159 161 162 163 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 167 169 169 172 178 180 183 183 184 6 Volumetric analysis 6.1 Prior knowledge . . . . . . . . . . 6.2 Introduction . . . . . . . . . . . . 6.3 Quality control . . . . . . . . . . . 6.4 Practical applications of titrations 6.5 Summary . . . . . . . . . . . . . . 6.6 Resources . . . . . . . . . . . . . 6.7 End of topic test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 189 191 192 194 194 198 198 199 7 End of unit test 201 Glossary 216 Hints for activities 219 Answers to questions and activities 1 Getting the most from reactants 2 Chemical equilibria . . . . . . . 3 Chemical energy . . . . . . . . 4 Oxidising or reducing agents . 5 Chromatography . . . . . . . . 6 Volumetric analysis . . . . . . . 7 End of unit test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 220 220 234 238 246 254 258 261 © H ERIOT-WATT U NIVERSITY 1 Topic 1 Getting the most from reactants Contents 1.1 Prior knowledge . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 1.2 1.3 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . The design of industrial chemical processes . . . . . . . . . . . . . . . . . . . 4 5 1.4 Mole calculations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.4.1 The mole . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 10 1.5 1.4.2 Avogadro's constant . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Molar volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15 22 1.6 1.7 Reacting volumes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Percentage yields . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28 32 1.8 1.7.1 Calculations involving percentage yields . . . . . . . . . . . . . . . . . . The atom economy of a process . . . . . . . . . . . . . . . . . . . . . . . . . . 35 37 Excess . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.9.1 Calculating excess . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43 43 1.10 Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.11 Resources . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46 47 1.12 End of topic test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48 1.9 Prerequisite knowledge Before you begin this topic, you should know or be able to: • understand and explain the factors affecting rates of reaction (Higher, Unit 1); • to compare rates of chemical reactions, changes in mass, volume and other quantities can be measured. Graphs can then be drawn to help this comparison (National 4, Unit 1); • the gram formula mass is defined as the mass of one mole of a substance (National 5, Unit 1); • use the chemical formula of any substance the gram formula mass can be calculated using relative formula masses of its constituent elements (National 5, Unit 1); • the concentration of solutions in moles per litre (National 5, Unit 1); 2 TOPIC 1. GETTING THE MOST FROM REACTANTS • calculations to determine the concentration and volume and the mass of a substance through the number of moles present (National 5, Unit 1); Learning objectives At the end of this topic, you should know that: The chemical industry • Industrial processes are designed to maximise profit and minimise the impact on the environment. • Factors influencing process design include: availability, sustainability and cost of feedstock(s); opportunities for recycling; energy requirements; marketability of byproducts; product yield. • Environmental considerations include: minimising waste; avoiding the use or production of toxic substances; designing products which will biodegrade if appropriate. Chemical calculations • Balanced equations show the mole ratio(s) of reactants and products. Using the balanced equation and the gram formula masses (GFM), mass to mass calculations can be performed. • The quantity of a reactant or product can also be expressed in terms of moles. The concentration of a solution can be expressed in mol l -1 . • Balanced equations can be used in conjunction with concentrations and volumes of solutions and/or masses of solutes to determine quantities of reactants and/or products. Molar volume • The molar volume (in units of litres mol -1 ) is the same for all gases at the same temperature and pressure. The volume of a gas can be calculated from the number of moles and vice versa. • The volumes of reactant and product gases can be calculated from the number of moles of each reactant and product. Percentage yield • The efficiency with which reactants are converted into the desired product is measured in terms of the percentage yield and atom economy. • Percentage yields can be calculated from mass of reactant(s) and product(s) using a balanced equation. • Given costs for the reactants, a percentage yield can be used to calculate the feedstock’s cost for producing a given mass of product. © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS Atom economy • The atom economy measures the proportion of the total mass of all starting materials successfully converted into the desired product. • It can be calculated using the formula shown below in which the masses of products and reactants are those appearing in the balanced equation for the reaction. • Atom Economy = (mass of desired product(s) / total mass of reactants) × 100. • Reactions which have a high percentage yield may have a low atom economy value if large quantities of unwanted by-products are formed. Excess • In order to ensure that costly reactant(s) are converted into product, an excess of less expensive reactant(s) can be used. • By considering a balanced equation, the limiting reactant and the reactant(s) in excess can be identified. • Whilst the use of excess reactants may help to increase percentage yields, this will be at the expense of the atom economy so an economic / environmental balance must be struck. © H ERIOT-WATT U NIVERSITY 3 4 TOPIC 1. GETTING THE MOST FROM REACTANTS 1.1 Prior knowledge Test your prior knowledge Q1: Go online a) b) c) d) Which three variables can be altered to change the rate of a reaction? Concentration Particle Size Temperature All of the above .......................................... Calculate the mass, in grams, of 0.25 moles of Butane (C 4 H10 ). Q2: a) b) c) d) 58 56 29 14.5 .......................................... Q3: 10.0 cm3 of potassium hydroxide (KOH) of concentration 0.25 mol l -1 is titrated with nitric acid. It takes exactly 12.5 cm3 of the acid to neutralise the alkali. Calculate the concentration of the nitric acid. 0.1 mol l-1 0.2 mol l-1 1.0 mol l-1 2.0 mol l-1 a) b) c) d) .......................................... 1.2 Introduction The chemical industry is a major contributor to the British, and Scottish, economy. This unit considers the principles governing chemical reactions. There are four major sections: • Stoichiometry and equilibrium; • Kinetics; • Energy; • Chemical analysis. Why is this topic important? • The oldest site for manufacturing chemicals in Scotland was established in 1871 and is still operating today. • More than 13,500 people are employed in the chemical industry in Scotland. © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS • The chemical industry generates a turnover of £3.4 billion annually. • Scotland is part of the EU - the world’s largest chemicals market both as a producer and consumer. After a look at general factors which influence the design of industrial processes, this topic looks at stoichiometry, the relationship between the quantities of reactants and products, and studies equilibrium in reversible reactions. 1.3 The design of industrial chemical processes Key point Industrial processes are designed to maximise profit and minimise the impact on the environment. "The Chemical Industry is not in existence to manufacture chemicals: like any other industry it exists to create wealth and wealth can only be created if it can make profits." So, the major determinant in any process design is how do we make the most profit from our product, to enable investment in future products, and with due regard to the safety of the workers, the local community and protecting the environment? Until a few decades ago concern for the wider environment was not given much consideration. Nowadays, the safety and environmental effects of the manufacture, use and disposal of consumer products, including chemicals, is of paramount importance. Many chemical processes are changing in response to the requirements of 'green chemistry'. You will look at some of these later in this topic. Process design economics In order to maximise the profitability of a chemical process, several factors have to be considered. • Raw materials and feedstocks have to be: ◦ readily available; ◦ preferably from sustainable sources; ◦ and at a suitable cost. • Recycling of materials should be able to reduce costs, compared with new manufacture. • The energy requirements for a process should be minimised. • Can any unavoidable by-products also be sold at a profit or do they have to be disposed of as costly waste? • The process should have the highest possible yield and the maximum atom economy. (These will be discussed in detail later.) © H ERIOT-WATT U NIVERSITY 5 6 TOPIC 1. GETTING THE MOST FROM REACTANTS Sulfuric acid manufacture The figure below shows a sulfuric acid manufacturing plant. The corresponding flow chart is shown below. Sulfuric acid plant at ICI Billingham, UK © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS Q4: Look at the flow chart for the production of sulfuric acid. What are the feedstocks for this process? a) b) c) d) Sulfur, Air and Water. Sulfur, Air, Sulfur Trioxide and Water. Sulfur and Air. Air and Water. .......................................... Q5: What are the advantages of using these materials as feedstocks? a) Readily available. b) Sustainably sourced. c) Available at a suitable cost. d) No toxic by-products produced. e) Waste by-products can be sold on. .......................................... Q6: You can see that there are three passes of the SO 2 /O2 mixture through a series of catalyst beds and coolers. Why do you think this is? a) To make the reaction more economical. b) To neutralise products. c) Catalysts can be used over and over again. d) So that no toxic by-products produced. .......................................... Q7: Does this process generate wasteful by-products? a) Yes b) No .......................................... Q8: Can you see anywhere where energy saving can be used? .......................................... .......................................... Safety The handling of chemicals is increasingly controlled by regulations such as COSHH (Control of Substances Hazardous to Health Regulations, 1999), where the hazards are clearly defined and measures to ensure safety are implemented before any contact with the chemicals takes place. Part of this involves all chemicals being clearly labelled with an EU Hazard symbol (some of these are shown below). © H ERIOT-WATT U NIVERSITY 7 8 TOPIC 1. GETTING THE MOST FROM REACTANTS ! Environmental considerations These are largely achieved by designing processes which: • minimise the production of waste; either as unwanted products, or through the use of materials (e.g. solvents) which require disposal at the end of the process; • and avoid the use or production of toxic materials, or those which will not degrade in the environment. DDT The insecticide DDT was a very effective agent against malaria-carrying mosquitos. It has several advantages: • it is cheap to produce; • it is effective against a wide variety of insect pests; • and it is relatively non-toxic to mammals. The incidence of the cases of malaria dropped very significantly in the 1970s following widespread spraying in countries such as India (with 500,000 deaths from malaria in 1960, but only 1000 each year in the early 1970s). © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS Crop spraying in East Germany (http:/ / bit.ly/ 2bk47 V p, by http:/ / bit.ly/ 2bk24lk) Deutsches Bundesarchiv (German Federal Archive), Bild 183-20820-0001. http:/ / cr e ativecommons.or g/ licenses/ by-sa/ 3.0/ d e/ d eed .d e Its stability, an advantage to its use, also meant that it would be able to persist for long periods in the environment. DDT has even been carried to and detected in the Antarctic, where it has never been used. It also increases in concentration along the food chain. A consequence of this was the thinning of bird's egg shells, which broke before hatching, resulting in alarming drops in the population of birds of prey like sparrowhawks and peregrine falcons. Nowadays the use of DDT is banned in many countries. Use the internet to investigate new insecticides. Are they as effective at destroying pests? What is their expected 'lifetime' in the environment? .......................................... In many cases the problems associated with chemicals in the environment were not anticipated when the chemical was first used. Chemical companies can solve these problems by developing improved products, for example: • Hydrogen-containing CFCs, called HCFCs, are refrigerants without the ozone-damaging chlorine atoms. The importance of changing to alternative chemicals is described in the Montreal Protocol on Substances that Deplete the Ozone Layer, which came into effect in 1989. • Modern insecticides do not persist in the environment, so do not cause the problems of DDT. The trade-off is that they are expensive and therefore not used extensively by the poorer countries, where the incidence of malaria is increasing. © H ERIOT-WATT U NIVERSITY 9 10 TOPIC 1. GETTING THE MOST FROM REACTANTS • Alternative and renewable sources of energy, which do not produce CO 2 (e.g. hydrogen and wind or wave generated electricity) are replacing fossil fuels. The Kyoto protocol, in 1997, was an attempt by world governments to reduce the output of CO2 , and so reduce global warming effects. 1.4 Mole calculations Whether you are carrying out a small scale preparation of a few grams of material in a laboratory or producing hundreds of tonnes of a commercial product, it is vital that you can calculate the amounts of reactants required and the amount of product(s) you expect. In order to do this you have to be able to balance chemical equations. All chemical calculations depend on correctly balanced equations. If you are unsure about balancing chemical equations you should practise now. 1.4.1 The mole At National 5 level, one mole of a substance was defined as the formula mass expressed in grams (i.e. the gram formula mass - GFM). The number of moles is therefore a measure of the amount of substance. Key point The mole is fundamental to nearly all calculations in chemistry. The equation below shows a balanced equation for the formation of carbon monoxide from carbon and carbon dioxide, one of the reactions which occurs in a blast furnace, used for making iron. Formation of carbon monoxide One interpretation of this equation is that one atom of carbon reacts with one molecule of carbon dioxide to form two molecules of carbon monoxide. This is not much help to a chemist since it is impossible to measure out individual atoms and molecules. A better interpretation is that one mole of carbon and one mole of carbon dioxide react to form two moles of carbon monoxide (see below). Formation of CO with mole relationships These are real quantities which can be measured. Notice that the total mass on the left of the equation equals the total mass on the right. The mole provides a link between © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS atoms and molecules reacting at the atomic level and chemical reactions occurring in the 'real' world. Mole display The figure above shows one mole of each of four substances. Note that although the amount of substance is the same (same number of moles), the mass of each substance is different because the individual atoms and molecules making up each substance have different masses. Mass calculations You should be familiar with calculations involving reacting masses from previous chemistry courses, so here is a short exercise to check your knowledge. You will need the SQA data booklet to obtain relative atomic masses. Q9: 'Fools' gold' is a mineral with formula FeS 2 . A piece of the mineral contains 13.95 g of iron, how many grams of sulfur are present? a) b) c) d) 13.95 16.05 27.90 32.10 .......................................... Q10: Iron disulfide, FeS 2 can be used as a source of sulfur for sulfuric acid manufacture. The ore is roasted in air to produce sulfur dioxide and iron(iii) oxide. Which is the balanced equation for this reaction? a) b) c) d) FeS2 + 3O2 → FeO2 + 2SO2 2FeS2 + 5O2 → Fe2 O3 + 4SO2 4FeS2 + 11O2 → 2Fe2 O3 + 8SO2 6FeS2 + 16O2 → 4Fe2 O3 + 16SO2 .......................................... Q11: 204 tonnes of iron disulfide ore was heated in air. How many tonnes of sulfur dioxide were produced? © H ERIOT-WATT U NIVERSITY 11 12 TOPIC 1. GETTING THE MOST FROM REACTANTS a) b) c) d) 204.0 217.9 256.4 512.8 .......................................... Q12: You need to make 100 kg of sulfuric acid. Assuming total conversion at each stage, how many kg of FeS 2 would you need to start with? a) b) c) d) 244.65 122.32 100.00 61.16 .......................................... Q13: You are going to prepare 100 g of the ester methyl benzoate. Assuming complete conversion, how many grams of methanol and benzoic acid would you require? .......................................... Calculations involving solutions Many chemical reactions are carried out in solution, so you have to be able to handle calculations with solutions. Chemists often express the concentration of solutions in mol -1 . You actually weigh out grams of solutes, so you have to be able to convert mol -1 to g -1 and vice versa. Here are some examples of calculation questions with solutions. You might like to try to answer them yourself first, then check your solution (pun intended!). After these examples there is a series of questions for you to try. Example : Mass to concentration What is the concentration, in mol -1 , of a solution of sodium chloride made by dissolving 9 g of NaCl in 1 litre of solution? 1. Calculate the GFM (gram formula mass) of sodium chloride: NaCl is 23.0 + 35.5 = 58.5 2. Calculate the number of moles: 58.5 g is 1 mol 1 g is 1/58.5 mol 9 g is 9/58.5 mol = 0.154 mol (to 3 decimal places) 3. Calculate the concentration: moles concentration = volume (in litres) concentration = 0.154 1 = 0.154 mol -1 to 3 decimal places .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS Examples 1. Mass to concentration What is the concentration, in mol -1 , of a solution of silver nitrate made by dissolving 2.30 g of AgNO 3 in 100 ml of solution? 1. Calculate the GFM (gram formula mass) of silver nitrate: AgNO3 is 107.9 + 14.0 + (3 × 16.0) = 169.9 2. Calculate the number of moles: 169.9 g is 1 mol 1 g is 1/169.9 mol 2.30 g is 2.3/169.9 mol = 0.0135 mol (to 3 significant figures) 3. Change the volume to litres: 100 ml = 0.1 4. Calculate the concentration: concentration = 0.0135 0.1 = 0.00135 = 0.00135 mol -1 to 3 significant figures .......................................... 2. Concentration to mass How many moles and grams are in 250 ml of solution of calcium bromide of concentration 0.15 mol -1 ? 1. Write the formula of calcium bromide, then calculate the GFM: Formula is CaBr2 GRM is 40.0 + (2 ×79.9) = 199.8 2. Change volume to litres 250 ml = 0.25 3. Calculate number of moles of solute: moles of solute = concentration (in mol -1 ) × volume (in litres) moles = 0.15 × 0.25 = 0.0375 mol 4. Calculate number of grams of solute: 1 mol = 199.8 g 0.0375 mol = 0.0375 × 199.8 = 7.49 g to 3 sig. figures .......................................... 3. Reaction - masses and solutions What mass of magnesium, in grams, can react completely with 100 ml of 0.25 mol -1 hydrochloric acid? 1. Write a balanced equation: Mg + 2HCl → MgCl2 + H2 From which: 1 mol Mg reacts with 2 mol HCl © H ERIOT-WATT U NIVERSITY 13 14 TOPIC 1. GETTING THE MOST FROM REACTANTS 2. Calculate the number of moles of HCl (what you know): moles of solute = concentration (in mol -1 ) × volume (in litres) moles = 0.25 × 0.10 = 0.025 mol 3. Calculate number of moles, and grams, of Mg reacting with this amount of HCl: 2 mol HCl reacts with 1 mol Mg 0.025 mol HCl reacts with 0.025/2 mol Mg = 0.0125 mol 4. Atomic mass of magnesium is 24.3 so mass of Mg: 0.0125 mol Mg = 0.0125 × 24.3 = 0.30375 g .......................................... 4. Reaction - masses and solutions A chemist makes a solution containing copper(II) ion by dissolving 49.92 g hydrated copper(II) sulfate (CuSO4 .5H2 O) in 500 ml of solution. What is the concentration of this in mol -1 ? Zinc metal, 3.27 g, was added to 200 ml of the copper sulfate solution. What mass, in grams, of copper(II) ion was left in solution after reaction with the zinc? Copper sulfate solution is 0.40 mol -1 200 ml of this contains 0.08 mol Cu 2+ ion 3.27 g Zn is 0.05 mol from the equation 1 mol Zn reacts with 1 mol Cu 2+ , so 0.03 mol Cu2+ left in solution 1.905 g copper ion is left in solution .......................................... 5. Reaction - solutions Insoluble barium sulfate can be prepared by adding solutions containing a soluble barium salt and a soluble sulfate. A solution of barium chloride (BaCl 2 ) contains 104.15 g in 1 litre. What volume of sodium sulfate (Na2 SO4 ) containing 142.1 g in 1 litre, would be required to react completely with 50 ml of the barium chloride solution? 1. Write a balanced equation: BaCl 2 (aq) + Na2 SO4 (aq) → BaSO4 (s) + 2NaCl(aq) 2. Calculate the relative formula masses: BaCl 2 - 208.3; Na2 SO4 - 142.1 3. Calculate the concentrations of solutions: BaCl 2 - 104.15/208.3 = 0.5 mol -1 ; Na2 SO4 - 142.1/142.1 = 1.0 mol -1 4. 50 ml of barium chloride contains 0.05 × 0.5 = 0.025 mol 5. 1 mol BaCl2 reacts with 1 mol Na2SO4, so 0.025 mol BaCl 2 reacts with 0.025 mol Na2 SO4 . 6. Concentration of Na 2 SO4 is 1.0 mol -1 , so 0.025 mol is contained in 0.025 = 25 ml. .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS 1.4.2 15 Avogadro's constant Note: Avogadro’s Constant is a suggested learning activity, not a key area of the course. To understand what one mole of water and one mole of sodium chloride have in common, we can use a simple analogy. Q14: How many nails are on the right hand balance? .......................................... In this example, the number of nails is small enough to be counted. However, this is not feasible with very large numbers. Suppose you are a manufacturer of nuts, bolts and washers. It is essential to manufacture the same number of nuts and bolts since one is useless without the other. Nuts and bolts Figure 1.1: Nuts and bolts Go online .......................................... Q15: How many nuts are in the pile of nuts? (Note that one tonne = 1000 kg.) .......................................... Q16: How many bolts are in the pile of bolts? .......................................... Q17: The manufacturer wants the same number of washers. What mass in tonnes does he need? .......................................... © H ERIOT-WATT U NIVERSITY 16 TOPIC 1. GETTING THE MOST FROM REACTANTS Key point It is possible to count very large numbers of small objects by weighing them. .......................................... A similar approach can be used with atoms as shown in the next activity. Avogadro's constant calculations Figure 1.2: Avogadro's constant Go online mass of one atom mass of one atom Number of atoms He 4.0amu 4.0 g C 12.0amu 12.0 g L ? Al 27.0amu ? .......................................... Q18: On paper, write an expression for L, the number of atoms in one mole of helium. (If you have difficulty, think back to the 'Nuts and bolts' activity. How did you work out the number of nuts in the pile?) .......................................... Q19: Using Figure 1.2, how many atoms of carbon are in one mole of carbon? .......................................... Q20: What mass in grams of aluminium will contain the same number of atoms? Give your answer to 1 decimal place. .......................................... The number of atoms in one mole of any element is known as Avogadro's constant which is given the symbol L. It has a value of 6.02 × 10 23 mol-1 . (Care must be taken with elements such as oxygen and the halogens which are diatomic.) This number is so enormous that it is very difficult to fully comprehend it. A number of analogies have been developed to try to help, e.g. • In order to get 6.02 × 10 23 grains of sand, an area the size of the Sahara Desert would have to be excavated to a depth of 2 metres. • Try to work the next one out for yourself. Q21: If a 1p coin is 1 mm thick, how far would a column of 6.02 × 10 23 1p coins stretch? Give your answer in light years, to three significant figures, where one light year is the distance travelled by light in one year. (The speed of light = 3 × 10 8 m s-1 .) .......................................... After the Sun, the nearest star to planet Earth is just over 4 light years away! Avogadro's constant is a huge number which in turn means that individual atoms are incredibly small. © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS 17 Key point One mole of helium (4.0 g) contains the same number of atoms as one mole of carbon (12.0 g) and one mole of aluminium (27.0 g). This number is known as Avogadro's constant (symbol L) and has a value of 6.02 × 10 23 mol-1 . .......................................... So, Avogadro's constant is the number of atoms in one mole of an element, except for those elements which exist as diatomic molecules. What about compounds like sodium chloride which contain ions rather than atoms? Formula units Figure 1.3: Formula units Go online .......................................... Q22: Complete the following sentence: In diamond, one formula unit is a carbon ....... .......................................... Q23: Complete the following sentence: In oxygen, one formula unit is an oxygen ....... .......................................... © H ERIOT-WATT U NIVERSITY 18 TOPIC 1. GETTING THE MOST FROM REACTANTS Q24: In sodium chloride, one formula unit is: a) a sodium ion. b) a chloride ion. c) one sodium ion and one chloride ion. .......................................... Q25: In silicon dioxide, one formula unit is: a) b) c) d) one silicon atom and one oxygen atom. one silicon(IV) ion and one oxide ion. one silicon atom and two oxygen atoms. one silicon(IV) ion and two oxide ions. .......................................... Key point The term 'formula unit' is a general term which relates to the type of particles which make up a substance. In general, it refers to the formula normally used for the substance. .......................................... In general: • The formula unit is an atom for all elements which do not exist as diatomic molecules, e.g 4.0 g of helium 31.0 g of phosphorus 55.8 g of iron All contain L atoms (6.02 × 10 23 atoms) • The formula unit is a molecule for all diatomic elements and covalent molecular compounds, e.g. 32.0 g of oxygen 44.0 g of carbon dioxide 18.0 g of water All contain L molecules (6.02 × 10 23 molecules) • For all ionic compounds and covalent network compounds, the formula unit is the simplest ratio of atoms or ions in the formula of the compound, e.g. for sodium chloride © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS Molar quantity 58.5 g of Na+ Cl- Number of formula units L (6.02 × 1023 ) Number of Na+ ions L Number of • 19 Cl- ions L Total number of ions 2L e.g. for silicon dioxide Molar quantity 60.1 g of SiO2 Number of formula units L (6.02 × 1023 ) Number of Si atoms L Number of O atoms 2L Total number of atoms 3L A more general definition of the mole and Avogadro's constant can now be given. Key point One mole of any substance contains 6.02 × 10 23 formula units. This number is known as Avogadro's Constant (L). Equimolar quantities of substances contain equal numbers of formula units. It is very unusual to use exactly molar quantities of substances. However any mass of substance can be quoted as a number of moles by using the relationship: Number of moles = mass in grams mass in grams or GFM mass of 1 mole Since one mole of any substance contains L formula units, it follows that equimolar quantities (quantities containing the same number of moles) must contain the same number of formula units, e.g. 0.1 moles of each of the following substances will contain the same number of formula units. Substance Mass of 0.1 mole Number of formula units oxygen 3.2 g 0.1 × L molecules iron 5.58 g 0.1 × L atoms sodium chloride 5.85 g 0.1 × L NaCl units © H ERIOT-WATT U NIVERSITY 20 TOPIC 1. GETTING THE MOST FROM REACTANTS The mole is being used as a counting unit. There are many other examples of names being given to counting units, e.g. dozen, gross, ream (500 sheets of paper) etc. Calculations using Avogadro's constant (optional) Go online At National 5 level, calculations involving the conversion of mass to moles and moles to mass were covered. At Higher another stage is added: Type A Mass → number of moles → number of particles Type B Number of particles → number of moles → mass Note that the number of moles is central to these calculations. These calculations are much easier to manage if a standard routine is used each time a calculation is carried out. Examples 1. A - from mass to number of particles A glass of water contains 210 g of water. 1. How many molecules of water are there in the glass? 2. What is the total number of atoms in the water? The first step is to work out the direction of the calculation, i.e. what information have you been given and what are you asked to find out. Always write the direction as shown below with 'Given' to the left and 'Required' to the right. Then, below that write the 'Key relationship' in the same order. 1. Now you can start to use the information from the question. The gram formula mass of water is 18 g. Given Required Mass of water Number of molecules Key relationship 1 mole contains L formula units For water 18 g contains 6.02 × 1023 molecules In this case 210 g contains 210/18 × 6.02 × 10 23 molecules Answer 210 g water contains 7.02 × 1024 molecules Here is the calculation: 18 g of water contains 6.02 × 10 23 molecules By proportion, © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS 21 6.02 × 1023 molecules 18 23 210 × 6.02 × 10 molecules 18 1 g of water contains 210 g contains = 70 × 1023 molecules = 70 × 1024 molecules 2. One H2 O molecule contains two H atoms and one O atom, i.e. 3 atoms in one molecule. Total number of atoms = 3 × 7.0 × 1024 = 2.1 × 1025 .......................................... 2. B - Particles to mass What mass of silicon dioxide would contain 1.204 × 10 21 atoms of silicon? As before, work out the direction of the calculation and then write the key relationship beneath it with the 'Given' to the left and the 'Required' to the right. One formula unit of silicon dioxide is SiO 2 , i.e. one formula unit contains one Si atom. Given Required Number of Si atoms Mass of silicon dioxide Key relationship L formula units weigh 1 mole For SiO2 6.02 × 1023 formula units weigh 60.1 g For SiO2 6.02 × 1023 Si atoms are in 60.1 g SiO 2 In this case 1.204 × 1021 Si atoms (≡ to 0.01204 × 10 23 ) are in 60.1 × 0.01204/6.02 g Answer 1.204 × 1021 Si atoms are in 0.1202 g SiO 2 Here is the calculation: 6.02 × 1023 atoms of Si in one GFM 6.02 × 1023 atoms of Si in 60.1 g of SiO 2 By proportion, 1 atom in 60.1 g 6.02 × 1023 of SiO2 1.204 × 1021 atoms in 60.1 × 1.204 6.02 60.1 × 1.204 × 1021 6.02 × 1023 g 10−2 g = 12.02 × 10-2 g mass of SiO2 = 0.1202 g .......................................... © H ERIOT-WATT U NIVERSITY 22 TOPIC 1. GETTING THE MOST FROM REACTANTS Try the next five questions for yourself. Write your answers and working on paper before revealing the answers. All have worked answers which you can use as a check if you have difficulty. Q26: How many molecules are there in 0.22 g of carbon dioxide? .......................................... Q27: What mass of calcium carbonate would contain 3.612 × 10 20 formula units? .......................................... Q28: a) How many molecules are present in 0.112 g of methane? b) How many atoms are present in 0.112 g of methane? .......................................... Q29: How many ions are present in 37kg of magnesium nitrate? .......................................... Q30: What mass of potassium sulfate would contain 9.03 × 10 21 ions? .......................................... .......................................... 1.5 Molar volume When measuring out quantities of solid reactants or products, it is usual to weigh them in a container of known mass. The mass of the solid can then be obtained by subtracting the mass of the container. Pure liquids can be weighed in a similar way. The masses obtained can be converted to molar quantities using the GFM of the substance. However, gases are very difficult to weigh since they have very low densities and tend to spread easily, not to mention that most of them are invisible! It is more appropriate to measure the volume of a gas. However, there are problems. The volume of a gas depends on the temperature of the gas and also on the pressure. A balloon in front of a fire expands as it gets hotter and hotter and the pressure inside increases until the balloon bursts. So the number of gas molecules in a one litre sample will depend on the conditions of temperature and pressure. e.g. at different temperatures, © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS fewer molecules 23 more molecules e.g. at different pressures, more molecules fewer molecules A valid comparison between volumes of gases can only be made if the conditions of temperature and pressure are the same. The following activity shows how the volume occupied by one mole of a gas can be calculated. This volume is known as the molar volume. © H ERIOT-WATT U NIVERSITY 24 TOPIC 1. GETTING THE MOST FROM REACTANTS Molar volume Go online Example : Calculating the molar volume Using the apparatus shown, 1 of methane was collected. The initial reading on the balance was 124.86 g and the final reading was 124.14 g. What is the molar volume of methane under these conditions? Results: Volume of methane collected = 1.0 l Initial mass = 124.86 g Final mass = 124.14 g Mass of methane released = 0.72 g Step 1 The molar volume is the volume occupied by one mole of methane. Formula of methane is CH4 . So one mole will weigh 16.0 g. Step 2 Identify the direction of the calculation. Direction of calculation is mass ⇒ volume 0.72 g occupy 1 l 1 l 1.0 g occupy 0.72 16.0 l 16.0 g occupy 0.72 = 22.2 l Molar volume of methane is 22.2 l mol −1 .......................................... Q31: Under these conditions, using the same apparatus with the same initial mass, 1 of hydrogen was collected. The final mass reading was 124.77 g. What is the molar volume of hydrogen? .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS Q32: Under these conditions, using the same apparatus with the same initial mass, 1 of carbon dioxide was collected. The final mass reading was 122.88 g. What is the molar volume of carbon dioxide? .......................................... Q33: What do you notice about the molar volumes of methane, hydrogen and carbon dioxide (under these conditions)? .......................................... Key point The molar volume is measured in units of mol -1 (also shown as dm3 mol-1 ) and is the same for all three gases since the conditions of temperature and pressure are the same. .......................................... Density Calculations The SQA data booklet shows the densities of selected elements including the gaseous elements. Note that the density is quoted in g cm -3 and measured at s.t.p. (standard temperature and pressure). Standard temperature for these measurements is 0 ◦ C and standard pressure is one atmosphere. It is a simple matter to calculate the molar volume of a gaseous element from its density. Example : Molar volume from density Calculate the molar volume of argon at s.t.p. From the data booklet: Density of argon = 0.0018 g cm -3 = 1.8 g l -1 (Since 1000 cm3 = 1 l) 1 mole of argon weighs 40.0 g Direction of calculation: mass ⇒ volume 1.8 g occupies 1.0 l 1.0 g occupies 1.0 1.8 40.0 g occupies l 40.0 1.8 = 22.22 l mol-1 So the molar volume of argon at s.t.p. is 22.22 l mol -1 Note that this means that for any gas molar volume = GF M density © H ERIOT-WATT U NIVERSITY 25 26 TOPIC 1. GETTING THE MOST FROM REACTANTS .......................................... Now you try. Calculate the molar volume for the following gases at s.t.p. using the values in the data booklet. Give your answers in mol -1 to 2 decimal places. Q34: Oxygen .......................................... Q35: Krypton .......................................... Q36: Chlorine .......................................... The cube in the photograph opposite represents the molar volume of helium at s.t.p. The density values in the data booklet have been rounded to 4 decimal places but even so it can be seen that the molar volume is about the same for all gases under these conditions (s.t.p.). This can be generalised further. Key point The molar volume is the same for all gases at the same temperature and pressure. Q37: Under particular conditions of temperature and pressure, the molar volume of neon is 30.0 mol -1 . Under the same conditions of temperature and pressure, what will be the molar volume of xenon in mol -1 ? .......................................... This leads to a very useful statement often referred to as Avogadro's hypothesis: 'Equal volumes of different gases, under the same conditions of temperature and pressure, contain the same number of molecules (atoms if the gas is a noble gas).' © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS 27 So provided that the temperature and pressure remain the same the volume of a gas is a measure of the number of moles of that gas. So the volume of a gas can be calculated from the number of moles and vice versa. Work carefully through the two examples and then try the questions which follow. Examples 1. Volumes to moles Under certain conditions, the molar volume of sulfur dioxide is 25.0 mol -1 . How many moles are present in 100 cm 3 of sulfur dioxide gas? Note that the units of molar volume are given in whereas the volume of SO 2 is given in cm3 . Before starting the calculation, the units must be made compatible. 1000 cm3 = 1 l So, 100 cm3 Direction of calculation: Volume ⇒ Moles 25.0 l = 1.0 mol 1.0 mol 1.0 l = 25.0 0.1 0.1 l = 25.0 = 0.004 mol .......................................... 2. Moles to volumes If the molar volume of carbon dioxide under certain conditions is 28.0 mol -1 , what volume in cm3 would be occupied by 0.04 mol? Direction of calculation: Moles ⇒ Volume 1.0 mol = 28.0 l 0.04 mol = 28.0 × 0.04 l = 1.120 l = 1120 cm3 .......................................... Molar volume - Further practice Click on Practice questions on molar volume to sit the test. Go online If you do not have access to the on-line version, try the following examples. Q38: Calculate the molar volume of hydrogen sulfide, in l mol -1 , if 0.7g occupies 320 cm3 . .......................................... © H ERIOT-WATT U NIVERSITY 28 TOPIC 1. GETTING THE MOST FROM REACTANTS Q39: How many moles of oxygen are there in 900 cm 3 if the molar volume is 28 l mol-1 ? .......................................... Q40: What volume is occupied by 0.59 moles of carbon dioxide if the molar volume is 28 l mol-1 ? .......................................... 1.6 Reacting volumes Provided that the conditions of temperature and pressure do not change, the volume of a gas can be used as a measure of the number of moles of the gas in the same way as the mass of a solid or pure liquid. This enables the volume of a gaseous reactant or product to be calculated from the appropriate balanced equation. Consider the electrolysis of water. The apparatus opposite can be used for the experiment. A small amount of sulfuric acid is added to the water to increase the conductivity and speed up the process. This does not affect the overall reaction. The gases produced at each electrode can be collected and the volume measured. © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS Example : Electrolysis of water In an experiment, 50 cm 3 of hydrogen is collected. What volume of oxygen would be collected at the same time? Start with the balanced equation and mole relationship: From the question, work out the direction of the calculation: All measurements are made at the same time and under the same conditions of temperature and pressure. The molar volumes of hydrogen and oxygen will be the same - equal volumes of gases will contain the same number of moles. 2 volumes of H2 1 volume of O2 2 cm3 1 cm3 50 cm3 25 cm3 .......................................... The following activity looks at the combustion of methane, in which both reactants and one of the products are gases. The combustion is carried out by mixing the methane and oxygen and igniting the mixture using an electric spark. After the reaction, the products and any unreacted reactants are allowed to cool back to room temperature. Provided that all measurements are made under the same conditions of temperature and pressure, the volumes of gases will be proportional to the number of moles present. © H ERIOT-WATT U NIVERSITY 29 30 TOPIC 1. GETTING THE MOST FROM REACTANTS Note that the volumes of liquids and solids can be ignored. The volumes of solids and liquids are negligible compared with equimolar volumes of gases as shown in the photograph opposite. Combustion of methane Go online Explanation Initial volume of oxygen = 50 cm3 Initial volume of methane = 20 cm 3 Final volume = 30cm3 © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS 31 Figure 1.4: Combustion of methane .......................................... Almost always, one of the reactants will be in excess. Before continuing, it is essential to establish which reactant is in excess. From the volume relationship (Figure 1.4), the volume of oxygen required for complete reaction is twice the volume of methane, i.e. 20 cm3 of methane would require 40 cm 3 of oxygen. The initial volume of oxygen is 50 cm3 and so the oxygen is in excess. From the equation, 20 cm3 of CH4 + 40 cm3 of O2 → 20 cm3 of CO2 volume of unreacted oxygen = 50 - 40 = 10 cm3 volume of CO2 produced = 20 cm3 So, the total volume of gas at the end = 30 cm 3 Note that if the temperature had been greater than 100 ◦ C, the water would have been present as steam (i.e. gas not liquid). The volume of the steam would have to be taken into account. From the mole relationship (Figure 1.4), 2 moles of H2 O are formed for every 1 mole of CH4 used up. So, 2 volumes of steam would also have been produced, an additional 40 cm 3 of gas. So the final volume would have been 70 cm 3 if all the measurements had been made at a temperature above 100 ◦ C. Questions of this type frequently arise in examinations. They are not as difficult as they may appear, provided you set them out in a logical order as shown above. .......................................... Key point The volume of gaseous products or gaseous reactants can be calculated from a balanced equation using the number of moles of a reactant or product. Combustion of methane - Further practice Try the next two questions for yourself before displaying the worked answers. A randomised test is available online. Further questions will appear in the tutorial at the end of the topic. © H ERIOT-WATT U NIVERSITY Go online 32 TOPIC 1. GETTING THE MOST FROM REACTANTS Q41: 200 cm3 of propane was exploded with 1300 cm 3 of oxygen. Calculate the volume and composition of the resulting gas mixture, assuming that all measurements were made at the same room temperature and pressure. .......................................... Q42: 400 cm3 of ethene was exploded with 900 cm 3 of oxygen. Calculate the volume and composition of the resulting gas mixture, assuming that all measurements were made at the same room temperature and pressure. .......................................... 1.7 Percentage yields In the industrial manufacture of a chemical, it is important to ensure that the process produces as much product as possible. The amount produced is known as the yield and this is usually expressed as a percentage yield. A 100% yield means that the maximum possible amount of product has been isolated. This amount is known as the theoretical yield. In practice, the theoretical yield is rarely, if ever, obtained for a variety of reasons: • some material is lost during purification; • there may be side reactions, i.e. more than one product; • the reaction may be reversible, i.e. only some of the reactants are converted into products. Balanced equations are used to calculate the theoretical yield. Example : Calculating the theoretical yield Consider the hydration of ethene to form ethanol. If 14 tonnes of ethene are reacted, what is the theoretical yield of ethanol? (Steam is assumed to be in excess.) First write a balanced equation for the reaction. Beneath the equation, write the mole relationship and beneath that insert the Gram Formula Mass (use the SQA data booklet). You only need to do this for the reactant and product stated in the question. Other reactants will be in excess. © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS 33 Theoretically, 28 g of ethene ⇒ 46 g of ethanol 28 tonnes ⇒ 46 tonnes 46 tonnes 1 tonne ⇒ 28 46 × 14 tonnes 14 tonnes ⇒ 28 = 23 tonnes The theoretical yield of ethanol is 23 tonnes. .......................................... If the actual mass of the product is known, then a percentage yield can be calculated. Example : From theoretical yield to percentage yield Following on from the last example, if only 20.7 tonnes of ethanol are produced, what is the percentage yield? The percentage yield is calculated by dividing the actual yield by the theoretical yield and multiplying by 100. percentage yield = actual yield × 100 theoretical yield (1.1) © H ERIOT-WATT U NIVERSITY 34 TOPIC 1. GETTING THE MOST FROM REACTANTS .......................................... In this example: percentage yield = 20.7 23.0 x 100 = 90% .......................................... If the percentage yield for a particular reaction is known, it is possible to predict the mass of product formed. Example : Predicting the mass of product Predict the mass of ethyl ethanoate produced when 12.0 g of ethanoic acid reacts with excess ethanol if the percentage yield is 60%. As always, start with the balanced equation, mole relationship and Gram Formula Mass (GFM). 60 g of ethanoic acid ⇒ 74 g of ethyl ethanoate 74 g of ethyl ethanoate 1 g of ethanoic acid ⇒ 60 74 × 12.0 12.0 g of ethanoic acid ⇒ 60 The theoretical yield is 14.8 g. The percentage yield was given as 60%. Rearranging Equation 1.1 gives: percentage yield × theoretical yield 100 60 × 14.8 = 100 = 8.88 g actual yield = © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS .......................................... 1.7.1 Calculations involving percentage yields This activity contains questions to give practice in calculations involving percentage yields. The first two questions have worked solutions. In the on-line version, these are followed by an on-line test which contains questions with random parameters which can be tried over and over again to build confidence. These questions also contain hints in the form of 'Steps'. Q43: Ethyne can be made in the laboratory by adding water to calcium carbide according to the above equation. 2.56 g of calcium carbide produced 0.99 g of ethyne. a) Calculate the theoretical yield of ethyne. b) Calculate the percentage yield in the reaction. .......................................... Q44: 1.3 g of ethyne reacts with an excess of chlorine to form 7.14 g of 1,1,2,2-tetrachloroethane. a) Calculate the theoretical yield of 1,1,2,2-tetrachloroethane. b) Calculate the percentage yield in the reaction. .......................................... © H ERIOT-WATT U NIVERSITY 35 36 TOPIC 1. GETTING THE MOST FROM REACTANTS Calculations involving percentage yields - Further practice Benzene is very important as an industrial feedstock. Go online Q45: 36 tonnes of benzene was converted into benzoic acid. Calculate the theoretical yield. Give your answer to one decimal place. .......................................... Q46: The actual yield was 51 tonnes. Calculate the percentage yield. Give your answer to one decimal place. .......................................... Primary alcohols can be oxidised to carboxylic acids according to the following equation. where R = the rest of the molecule. The following table shows four primary alcohols. © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS Compound R HCH3 - 1 2 3 C2 H5 C6 H5 - 4 Q47: If 11 g of compound 4 were oxidised, calculate the theoretical yield of acid. Give your answer to 2 decimal places. .......................................... Q48: If the percentage yield for the oxidation was 65%, calculate the actual yield of acid. Give your answer to 2 decimal places. .......................................... 1.8 The atom economy of a process Stoichiometry, equilibrium and percentage yield tell you a lot of vital information about the chemistry of a process, but they give no help in determining how good the process is to the environment. In the 1990s, Barry Trost of Stanford University in the USA devised a simple measure of how 'green' a particular process might be. The 'atom economy' of a process is defined as: It is sometimes stated as: how much of what you put into your pot ends up in your product. Anything you produce but do not need is waste and will have to be disposed of and this will add to the cost. Q49: Calculate the atom economy of the production of calcium oxide from calcium carbonate. The equation is: CaCO3 → CaO + CO2 .......................................... Q50: Calculate the atom economy of the production of sulfur trioxide from sulfur dioxide and oxygen. The equation is: SO2 + 1 /2 O2 → SO3 .......................................... Q51: What does an atom economy of 100% mean for the process? .......................................... Q52: What about rearrangement reactions? .......................................... © H ERIOT-WATT U NIVERSITY 37 38 TOPIC 1. GETTING THE MOST FROM REACTANTS Key point This exemplifies a general principle that: Addition reactions are preferable to elimination and substitution reactions, from an environmental angle. Ibuprofen The analgesic (pain killer) ibuprofen has the structure shown in Figure 1.5. It is illustrated in full structural formula and in skeletal form. In this activity you will consider the atom economy of the original and modern synthetic routes. In order to simplify the figures, skeletal structures will be used. Figure 1.5: Ibuprofen structure .......................................... Ibuprofen was invented by pharmacists at Boots in the 1960s. In order to allow companies to benefit financially from their research, at that time a patent was granted for several years during which time no other companies could manufacture or sell this product. BNC synthesis In the 1980s, the Boots' patent on production and sales expired and ibuprofen became licensed for over-the-counter sales. There was a rush to produce the pain-killer as cheaply as possible, using the most environmentally acceptable methods. A new company, BHC, now manufactures and sells ibuprofen. It uses an improved synthetic route with only three stages, as shown (Figure 1.6). © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS 39 Figure 1.6: Ibuprofen synthesis .......................................... Q53: The table below shows the reagents used in the new synthesis and how many of these atoms are incorporated into ibuprofen. Complete the remaining columns showing how many of these atoms are not used in the ibuprofen molecule and the masses used and discarded. Used in ibuprofen Reagent Not used Formula Mr Formula Mr Formula Mr C10 H14 134 C10 H13 133 ? ? C 4 H 6 O3 102 C2 H3 O ? ? ? H2 ? H2 ? ? CO ? CO ? ? Ibuprofen Total ? C13 H18 O2 ? Waste 206 ? ? .......................................... Q54: Using the relevant M r data in the complete table, calculate the percentage atom economy. .......................................... © H ERIOT-WATT U NIVERSITY 40 TOPIC 1. GETTING THE MOST FROM REACTANTS Q55: Look at the structures of the starting materials used to make ibuprofen in this 'green' method. Identify the atoms of these that end up in ibuprofen; circle the discarded atoms. .......................................... BOOTS' original process In the original process the same starting material was employed, but the synthetic process they devised had six stages. © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS Figure 1.7: Boots original synthetic route .......................................... Q56: One problem with syntheses that involve several steps is that the overall yield can be quite low. Suppose that each stage of this six-stage synthesis has a 90% yield. What is the overall yield in this case? .......................................... Q57: How does this compare with the overall yield in the 3-stage BHC process? (Assume that each stage has a 90% yield.) .......................................... More importantly, the process has a low proportion of raw materials and reagents actually ending up in the final product. © H ERIOT-WATT U NIVERSITY 41 42 TOPIC 1. GETTING THE MOST FROM REACTANTS The table below shows the reagents used in the synthesis, followed by a column showing how many of these atoms are used in the ibuprofen molecule and how many atoms are wasted. Used in ibuprofen Reagent Formula C10 H14 Mr Not used Mr 27 13 134 Formula C10 H13 C 4 H 6 O3 102 C2 H3 C4 H7 O2 Cl 122.5 CH C2 H5 ONa H3 O 68 0 19 0 NH3 O 33 0 H 4 O2 36 Total C20 H42 NO10 ClNa 514.5 133 Mr Formula H C 2 H 3 O3 1 75 C3 H6 O2 Cl 109.5 C2 H5 ONa H3 O 68 33 HO2 Ibuprofen 33 NH3 O H3 C13 H18 O2 206 Waste C7 H24 NO8 ClNa 19 3 308.5 Q58: Using data in this table, calculate the percentage atom economy. .......................................... About 3000 tonnes of ibuprofen are produced annually in the UK. (1 tonne = 1000 kg) Q59: A tablet of ibuprofen contains 200 mg. How many tablets of ibuprofen are taken each year in the UK? (Hint: take great care with the units mentioned. Manufactured amounts are in tonnes; tablets are in mg. 200 mg is 0.2 g) .......................................... Q60: The population of the UK is about 60 million. How many tablets is this per person per year? .......................................... Look at the two synthetic routes again (Figure 1.6 and Figure 1.7). You will have noticed that the latter five stages of the original synthesis have been replaced by two new methods for converting the -CO-CH 3 side chain to -CH(CH3 )-COOH in the BHC synthesis. Q61: What type of reactions are these new ones? .......................................... Q62: Explain how this improved synthetic route has achieved a higher atom economy. .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS You can read more about ibuprofen synthesis at the http://www.rsc.org/learn-chemistry/ resources/chemistry-in-your-cupboard/nurofen/1. http://www.rsc.org/learn-chemistry/resources/chemistry-in-your-cupboard/nurofen/1 .......................................... 1.9 Excess In chemical reactions, the reactants are often not present in quantities which react exactly. Some of one or more of the reactants may remain at the end of the reaction they are said to be in excess. (A common analogy is making sandwiches.) Q63: A loaf of bread contains 28 slices; a packet of cheese slices contains 12 slices. How many sandwiches can you make from these and what will remain? .......................................... 1.9.1 Calculating excess In the experiment on "Measuring rates" the reaction between zinc and hydrochloric acid eventually stopped giving off hydrogen, and observation showed that some zinc was left over. Revisit the simulation if you wish. The zinc is said to be "in excess" as all the hydrochloric acid has been used up. If the quantities present at the start of the reaction are known, the equation for the reaction can be used to calculate the excess quantity of zinc. Suppose the reaction started with 65.4 g of zinc and 500 cm 3 of 2 mol -1 hydrochloric acid. From the equation: 1 mole of zinc (65.4 g) reacts completely with 2 moles of hydrochloric acid. But 500 cm3 of 2 mol -1 hydrochloric acid contains only 1 mole of acid. Only half the zinc can react and 32.7 g would be left over (excess). Not all examples are so straightforward, however, and the worked example here shows a standard method of calculating which reactant is in excess and by how much. © H ERIOT-WATT U NIVERSITY 43 44 TOPIC 1. GETTING THE MOST FROM REACTANTS Problem Magnesium with a mass of 9.72 g is dropped into 300 cm 3 of 1 mol -1 sulfuric acid. Which reactant is in excess and by how much? Solution • Write a balanced equation • Choose one reactant and calculate the number of moles involved 24.3 g of magnesium is 1 mol 1 mol 1 g of magnesium is 24.3 9.72 × 1 mol 9.72 g of magnesium is 24.3 = 0.40 mol • Calculate quantities of the other reactant involved 1000 cm3 1 mol l −1 sulfuric acid contains 1 mol 1 mol 1 cm3 1 mol l −1 sulfuric acid contains 1000 300 × 1 mol 300 cm3 1 mol l −1 sulfuric acid contains 1000 = 0.30 mol • Use the balanced equation to work out which reactant is in excess In this case the magnesium and sulfuric acid react in a 1:1 ratio. Magnesium (present as 0.40 mol) is in excess by 0.10 mol. • Calculate excess present so 1 mol magnesium is 24.3 g 0.10 mol magnesium is 2.43 g (if required answer is in grams) © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS 45 Calculating excess - Tutorial examples The questions in this set have the first example with a worked solution available at the back of the book, the others have an answer given at the back of the book. Try the questions on paper. Use your data booklet to find values for relative atomic masses. Q64: The thermite process produces molten iron due to the heat given out in the reaction. It can be used to weld railway tracks together. An aluminium sample (50 g) is mixed with 100 g of iron (III) oxide and ignited. Calculate which reactant is in excess and by how much. .......................................... Q65: A 10 g piece of calcium carbonate is reacted with 500 cm 3 of 1 mol -1 hydrochloric acid. Name the reactant in excess. (Hint: remember to write a balanced equation.) .......................................... Q66: A 10g piece of calcium carbonate is reacted with 500 cm 3 1 mol -1 hydrochloric acid. Calculate the number of moles of excess. .......................................... Q67: The next three questions refer to this reaction. Zinc reacts with hydrochloric acid, giving hydrogen gas and a solution of zinc (II) chloride. When 1.962 g of zinc is used with 80 cm 3 1 mol -1 hydrochloric acid: Name the reactant in excess. .......................................... Q68: Write the number of moles of excess. (2 decimal places.) .......................................... Q69: What mass (in grams) of hydrogen gas will have been given off? (2 decimal places.) .......................................... Q70: The next four questions refer to this reaction. Copper (II) oxide powder with a mass of 1.693 grams is added to a beaker containing 50 cm3 of 0.25 mol -1 sulfuric acid. Name the reactant in excess. .......................................... Q71: The reaction beaker is now filtered. Which substance will be in the filter paper? .......................................... Q72: What mass (in grams) of excess reactant will be left? (2 decimal places.) .......................................... © H ERIOT-WATT U NIVERSITY Go online 46 TOPIC 1. GETTING THE MOST FROM REACTANTS Q73: The remaining solution is evaporated and dried. What mass of solid would be obtained? (1 decimal place.) .......................................... 1.10 Summary Summary The chemical industry • Industrial processes are designed to maximise profit and minimise the impact on the environment. • Factors influencing process design include: availability, sustainability and cost of feedstock(s); opportunities for recycling; energy requirements; marketability of by-products; product yield. • Environmental considerations include: minimising waste; avoiding the use or production of toxic substances; designing products which will biodegrade if appropriate. Chemical calculations • Balanced equations show the mole ratio(s) of reactants and products. • Using the balanced equation and the gram formula masses (GFM), mass to mass calculations can be performed. • The quantity of a reactant or product can also be expressed in terms of moles. • The concentration of a solution can be expressed in mol l -1 . • Balanced equations can be used in conjunction with concentrations and volumes of solutions and/or masses of solutes to determine quantities of reactants and/or products. Molar volume • The molar volume (in units of litres mol-1) is the same for all gases at the same temperature and pressure. • The volume of a gas can be calculated from the number of moles and vice versa. • The molar volume is the same for all gases at the same temperature and pressure. (approx. 24 l mol-1 ). • The volumes of reactant and product gases can be calculated from a balanced equation using the number of moles of each reactant and product. Percentage yield © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS Summary continued • The efficiency with which reactants are converted into the desired product is measured in terms of the percentage yield and atom economy. • Percentage yields can be calculated from mass of reactant(s) and product(s) using a balanced equation. • Given costs for the reactants, a percentage yield can be used to calculate the feedstock’s cost for producing a given mass of product. Atom economy • The atom economy measures the proportion of the total mass of all starting materials successfully converted into the desired product. • It can be calculated using the formula shown below in which the masses of products and reactants are those appearing in the balanced equation for the reaction. • Atom Economy = (mass of desired product(s) / total mass of reactants) × 100%. • Reactions which have a high percentage yield may have a low atom economy value if large quantities of unwanted by-products are formed. Excess • In order to ensure that costly reactant(s) are converted into product, an excess of less expensive reactant(s) can be used. • By considering a balanced equation, the limiting reactant and the reactant(s) in excess can be identified. • Whilst the use of excess reactants may help to increase percentage yields, this will be at the expense of the atom economy so an economic / environmental balance must be struck. 1.11 • Resources Higher Chemistry for CfE: J Anderson, E Allan and J Harris, Hodder Gibson, ISBN 978-1444167528 © H ERIOT-WATT U NIVERSITY 47 48 TOPIC 1. GETTING THE MOST FROM REACTANTS 1.12 End of topic test End of topic 1 test Go online This end of topic test is available online. If you do not have access to the internet, here is a paper version. Q74: The SQA data booklet contains information on the densities of elements at standard temperature and pressure (s.t.p.). Using this data, calculate the molar volume of oxygen in cm3 mol-1, giving your answer to 4 significant figures. .......................................... Q75: Identify the two gases which occupy the same volume. (Assume all measurements are made under the same conditions of temperature and pressure.) A) 7 g CO B) 32 g CH4 C) 4 g H2 D) 32 g SO2 E) 17 g NH3 .......................................... Q76: In which of the following reactions do the products have a lower volume than the reactants? A) CaCO3 (s) + 2HCl(aq) → CaCl2 (aq) + CO2 (g) + H2 O(l) B) C(s) + O2(g) → CO2 (g) C) CH4(g) + 2O2 (g) → CO2 (g) + 2H2 O(l) D) 2C(s) + O2 (g) → 2CO(g) .......................................... Hydrazine, N2 H4 , is a hydride of nitrogen used in rocket fuel. The balanced equation for the complete combustion of hydrazine is N 2 H4 (g) + 2O2 (g) → N2 (g) + 2H2 O(l) Q77: What volume of oxygen would be required for the complete combustion of 50 cm 3 hydrazine? .......................................... Q78: What would the final volume if a mixture of 50 cm 3 of hydrazine and 150 cm 3 of oxygen was ignited? © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS Assume that all measurements were made at 25 ◦ C and 1 atmosphere pressure. .......................................... Q79: Calculate the volume occupied by 0.80 g of hydrazine. Take the molar volume to be 24.0 l mol-1 . .......................................... Q80: Nitrogen monoxide reacts spontaneously when mixed with oxygen. 2NO(g) + O2 (g) → 2NO2 (g) How many litres of nitrogen dioxide could theoretically be obtained by mixing 5 litres of nitrogen monoxide and 2 litres of oxygen gas? (All volumes are measured under the same conditions of temperature and pressure.) A) B) C) D) 2 3 4 5 .......................................... Q81: The equation shows the reaction between magnesium ribbon and dilute hydrochloric acid. Mg(s) + 2HCl(aq) → MgCl2 (aq) + H2 (g) What volume of hydrogen will be produced when 1 g of magnesium is added to excess acid? (Take the molar volume of hydrogen to be 24.3 litres mol -1 .) A) B) C) D) 1.0 litre 2.0 litres 2.43 litres 24.3 litres .......................................... Trichorophenol (TCP) is an antiseptic which is used to relieve throat infections. It is prepared from phenol as follows: C6 H5 OH + 3Cl2 → C6 H2 Cl3 OH + 3HCl © H ERIOT-WATT U NIVERSITY 49 50 TOPIC 1. GETTING THE MOST FROM REACTANTS Q82: Calculate the percentage yield if 537 g of TCP is obtained from 300 g of phenol. Give your answer to the nearest unit. .......................................... Q83: Calculate the atom economy of this process. .......................................... Q84: Benzene, phenol and related compounds are most important as: A) face cream. B) feedstocks. C) fuels. D) fish food. E) .......................................... Q85: Excess marble chips (calcium carbonate) were added to 100 cm 3 of 1 mol l-1 hydrochloric acid. The experiment was repeated using the same mass of marble chips and 100 cm3 of 1 mol l-1 ethanoic acid. Which would have been the same for both experiments? A) The mass of marble chips left over after the reaction had finished. B) The time taken for the reaction to be completed. C) The rate at which the first 10 cm3 of gas was produced. D) The average rate of reaction. .......................................... Q86: Water from the Dead Sea contains 5 g l -1 of Br- ion. A bromine plant processes 1 million litres of Dead Sea water each hour, producing 4 tonnes of bromine. The percentage yield of the process is: A) 100% B) 80% C) 40% D) 8% .......................................... The feedstock for making ammonia by the Haber process must be purified. For example, carbon monoxide (CO) should not be present. © H ERIOT-WATT U NIVERSITY TOPIC 1. GETTING THE MOST FROM REACTANTS Q87: What are the features which can lead to improvements in the efficiency of a chemical process? A) Scrubbers B) Heat exchangers C) Improved catalysts D) Continuous E) Haber F) batch .......................................... Propanone, widely used as a solvent, is manufactured from cumene. Cumene is oxidised by air and the cumene hydroperoxide product is then cleaved. The mixture of propanone and phenol is separated by distillation. Q88: Complete the following flow chart to summarise the manufacture of propanone from cumene. © H ERIOT-WATT U NIVERSITY 51 52 TOPIC 1. GETTING THE MOST FROM REACTANTS .......................................... Q89: For every 10 tonnes of propanone produced in this industrial process, calculate the mass of phenol (C6 H5 OH) produced. Give your answer to 1 decimal place in tonnes. .......................................... Q90: Calculate the atom economy of this process. .......................................... Q91: Propanone can also be manufactured by the catalytic oxidation of propan-2-ol with oxygen. Write a balanced equation for this reaction. .......................................... Q92: Calculate the atom economy of this new process. .......................................... Q93: In industry, apart from atom economy, several factors influence the decision as to which route might be employed. Suggest three of these factors. .......................................... .......................................... © H ERIOT-WATT U NIVERSITY 53 Topic 2 Chemical equilibria Contents 2.1 Prior knowledge . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55 2.2 Reversible reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.3 Dynamic equilibrium . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56 57 2.4 Altering the equilibrium position . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.4.1 Changing temperature . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61 62 2.4.2 Changing pressure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.4.3 The influence of concentration . . . . . . . . . . . . . . . . . . . . . . . 63 65 2.4.4 The influence of catalysts . . . . . . . . . . . . . . . . . . . . . . . . . . 2.5 The Haber process . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67 67 2.5.1 Adjusting the concentration . . . . . . . . . . . . . . . . . . . . . . . . . 2.5.2 Temperature in the Haber process . . . . . . . . . . . . . . . . . . . . . 68 69 2.5.3 Pressure in the Haber process . . . . . . . . . . . . . . . . . . . . . . . 2.5.4 Catalysts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 70 71 2.6 Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.7 Resources . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 72 73 2.8 End of topic test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 74 Prerequisite knowledge Before you begin this topic, you should know or be able to: • understand and explain the factors affecting rates of reaction (Higher, Unit 1); • compare rates of chemical reactions, changes in mass, volume and other quantities can be measured. Graphs can then be drawn to help this comparison (National 4, Unit 1); • the gram formula mass is defined as the mass of one mole of a substance (National 5, Unit 1); • use the chemical formula of any substance the gram formula mass can be calculated using relative formula masses of its constituent elements (National 5, Unit 1); • the concentration of solutions in moles per litre (National 5, Unit 1); 54 TOPIC 2. CHEMICAL EQUILIBRIA • calculations to determine the concentration and volume and the mass of a substance through the number of moles present (National 5, Unit 1); • a very small proportion of water molecules will dissociate into an equal number of hydrogen and hydroxide ions (National 5, Unit 1). Learning objectives At the end of this topic, you should know that: Reversible Reactions • Many reactions are reversible, so products may be in equilibrium with reactants. Equilibrium • At equilibrium, the concentrations of reactants and products remain constant, but are rarely equal. • This may result in costly reactants failing to be completely converted into products. • In a closed system, reversible reactions attain a state of dynamic equilibrium when the rates of forward and reverse reactions are equal. Altering Position of Equilibrium • Le Chatelier's principle states that if the conditions of a chemical system at equilibrium are changed, the system responds by minimising the effect of the changes. • Changes in concentration, pressure and temperature can alter the position of equilibrium. The Haber Process • To maximise profits, chemists employ strategies to move the position of equilibrium in favour of products. • A catalyst increases the rate of attainment of equilibrium but does not affect the position of equilibrium. The effects of altering pressure, altering temperature, the addition or removal of reactants or products can be predicted for a given reaction. © H ERIOT-WATT U NIVERSITY TOPIC 2. CHEMICAL EQUILIBRIA 2.1 55 Prior knowledge Test your prior knowledge Q1: What is equilibrium? a) Equilibrium is a situation where forward and reverse reactions take place at the same rate. b) Equilibrium is a situation where forward and reverse reactions are at equal proportions. c) Equilibrium is a situation where forward reaction proceeds at a greater rate than the reverse reaction. d) Equilibrium is a situation where reverse reaction proceeds at a greater rate than the forward reaction. .......................................... Q2: What is a reversible reaction? a) A situation where forward and reverse reactions take place at the same rate. b) A reaction which proceeds in both directions at the same time. c) A reaction where the reverse reaction always proceeds at a greater rate than the forward reaction. d) A reaction where the forward reaction always proceeds at a greater rate than the reverse reaction. .......................................... Q3: Which of the following is a correct representation of the water equilibrium? a) b) c) d) H2 0(l) → H+ (aq) + OH- (ag) H2 0(l) ← H+ (aq) + OH- (ag) H2 0(l) = H+ (aq) + OH- (aq) H2 0(l) H+ (aq) + OH- (aq) .......................................... © H ERIOT-WATT U NIVERSITY Go online 56 TOPIC 2. CHEMICAL EQUILIBRIA 2.2 Reversible reactions Chemical reactions involve reactants changing into products and many reactions are not reversible. Frying an egg cooks the white and it is impossible to change it back into clear jelly (Figure 2.2). However some processes, including many chemical reactions are reversible. The products can be turned back into reactants. Consider a mixture of ice and water at 0◦ C (Figure 2.2). Some of the ice melts into water, while some of the water freezes into ice. These two processes can be represented by equations (Figure 2.1). Figure 2.1: Water freezing and melting .......................................... Figure 2.2: Irreversible and reversible processes Frying an egg is not a reversible process. The reaction progresses in a forward direction only. The melting of ice and freezing of water are reversible, and at 0◦ C are said to be in an equilibrium situation. .......................................... At 0◦ C the rates of melting and freezing become equal, and the ice and water are said © H ERIOT-WATT U NIVERSITY TOPIC 2. CHEMICAL EQUILIBRIA 57 to be in a state of equilibrium. The two equations in Figure 2.1 can be combined to represent the equilibrium situation (Figure 2.3). Figure 2.3: Water equilibrium .......................................... The double arrow represents the equilibrium. The two-way sign shows that the process can occur in either direction. This equation shows the equilibrium in a chemical system. Hydrogen and iodine can combine in the forward reaction to produce hydrogen iodide. At equilibrium, hydrogen iodide breaks up at the same rate to form hydrogen and iodine. Equilibrium of hydrogen iodide The on-line version of this reaction is animated. Go online .......................................... 2.3 Dynamic equilibrium System in equilibrium A system in equilibrium appears to be unchanging as far as an outside observer is concerned. The bottle of soda water or lemonade shown below has carbon dioxide dissolved in the water and also free carbon dioxide above the liquid. The system is closed so that nothing can enter or leave the container. © H ERIOT-WATT U NIVERSITY Go online 58 TOPIC 2. CHEMICAL EQUILIBRIA As some carbon dioxide in the gas dissolves, some carbon dioxide in the solution leaves to become gas. So long as the system remains closed there is a balance between the rates of the exchange. Notice that the concentrations at equilibrium are not necessarily equal. .......................................... The rates of forward and reverse reaction in this closed system are equal and the reversible reaction (Figure 2.4) has attained a dynamic equilibrium. This is the situation which applies in the closed container in the picture (left) but the other container is an open system (right), which cannot reach equilibrium because the CO2 is constantly escaping. Figure 2.4: carbon dioxide equilibrium © H ERIOT-WATT U NIVERSITY TOPIC 2. CHEMICAL EQUILIBRIA 59 .......................................... The forward reaction is described as being from left to right and the reverse reaction from right to left. Reversible reactions reach a point where they appear to stop and a balance between reactant and product concentrations (not usually equal) is formed. An example is the thermal (involves heat) breakdown of hydrated copper sulfate. Figure 2.5 .......................................... The dynamic nature of equilibrium .......................................... Go online A balance can be achieved in this open system by careful control over the rate of the forward and reverse reactions by control of the heating and the addition of water. An open system allows material to be lost to or gained by the surroundings. In a closed system reversible reactions reach a state of equilibrium when the rate of the forward reaction is equal to the rate of the reverse reaction. In a closed system no material is exchanged with the surroundings. The on-line version of this topic contains a simulation experiment establishing the reversible nature of the reaction. If you do not have access to the on-line material, this paragraph will explain what the simulation illustrates. Heating turns the blue copper sulfate crystals into white powder. The water is driven away leaving anhydrous copper sulfate. Anhydrous means "without water". The white anhydrous copper sulfate can be hydrated back in the reverse reaction by adding water. Hydrated means "with water". A balance can be achieved in this open system by careful control over the rate of the forward and reverse reactions by control of the heating and the addition of water. An open system allows material to be lost to or gained by the surroundings. In a closed system reversible reactions reach a state of equilibrium when the rate of the forward reaction is equal to the rate of the reverse reaction. In a closed system no material is exchanged with the surroundings. © H ERIOT-WATT U NIVERSITY 60 TOPIC 2. CHEMICAL EQUILIBRIA Establishing dynamic equilibrium Go online The on-line version of this topic contains an animated experiment which shows the molecular collisions occurring as hydrogen and iodine react and form a chemical equilibrium with hydrogen iodide. If you do not have access to the on-line material use this diagram of the situation at equilibrium to answer the questions. Q4: In the forward reaction, how many molecules of hydrogen and iodine (in total) are present before reaction starts? .......................................... Q5: In the forward reaction, how many reactant molecules of hydrogen and iodine are present when equilibrium has been established? .......................................... Q6: How many product molecules of hydrogen iodide are present when equilibrium has been established? .......................................... Q7: Which of these statements is correct? products at equilibrium: a) b) c) d) The concentrations of reactants and change rapidly to different concentrations. remain constant, but not necessarily equal. change slowly to equal concentrations. remain constant and equal. .......................................... Q8: Now look closely at the reverse reaction. How many product molecules of hydrogen iodide are present when equilibrium has been established? .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 2. CHEMICAL EQUILIBRIA Q9: Which of these statements is correct about the equilibrium mixture obtained when starting with hydrogen iodide rather than hydrogen and iodine? a) b) c) d) There is more HI than before. There is less HI than before. There is the same equilibrium mixture as before. There is less H2 and I2 than before. .......................................... Q10: Explain why this situation can be described as a dynamic equilibrium. .......................................... Key point A reversible reaction attains a state of dynamic equilibrium when the rates of forward and reverse reactions are equal. At equilibrium, the concentrations of reactants and products remain constant although not necessarily equal. The same equilibrium position is reached irrespective of whether starting from reactants or products. 2.4 Altering the equilibrium position It is particularly important that in any industrial process as much product as possible is produced. A process producing only small amounts of a required product in each cycle would be regarded as very inefficient, and would need modification to product more. This section looks at methods for influencing the equilibrium position. In a reaction system which is in chemical equilibrium the rate of the forward reaction is equal to the rate of the reverse reaction. At equilibrium, if there is a high proportion of products to reactants, the position of equilibrium is said to lie well to the right. Chemists often wish to influence the position of equilibrium to give a maximum yield of product. How changes in conditions can influence equilibria can be summarised in Le Chatelier's principle. Key point Le Chatelier's principle If the conditions of a chemical system at equilibrium are changed, the system responds by minimising the effect of the changes. Factors which influence the rate of a reaction, such as temperature, pressure, concentration and the addition of a catalyst might be expected to influence the position of equilibrium. © H ERIOT-WATT U NIVERSITY 61 62 TOPIC 2. CHEMICAL EQUILIBRIA 2.4.1 Changing temperature The influence of temperature The equilibrium between dinitrogen tetroxide and nitrogen dioxide is endothermic in the forward direction (see below). The reaction is therefore exothermic in the reverse direction. Because N2 O4 is colourless and NO2 is brown, the colour of the equilibrium mixture can give an indication of the equilibrium position. Equilibrium and temperature Go online The on-line version of this activity contains a simulation experiment in which the effect of temperature changes on the position of equilibrium can be investigated. If you do not have access to the on-line version, look at this diagram before answering the questions. .......................................... Q11: How many nitrogen dioxide molecules are present in the light brown equilibrium mixture at room temperature? .......................................... Q12: How many nitrogen dioxide molecules are present in the dark brown equilibrium mixture at the increased temperature? © H ERIOT-WATT U NIVERSITY TOPIC 2. CHEMICAL EQUILIBRIA .......................................... Q13: Has the equilibrium position shifted in the exothermic or endothermic direction? .......................................... Q14: Use Le Chatelier's principle to explain why this occurs. Write an answer before consulting the back of the text. .......................................... Q15: How many dark brown nitrogen dioxide molecules are present in the equilibrium mixture at the decreased temperature? .......................................... Q16: Has the equilibrium position shifted in the exothermic or endothermic direction? .......................................... Q17: Use Le Chatelier's principle to explain why this occurs. Write an answer before consulting the back of the text. .......................................... Key point Le Chatelier's principle can be used to predict changes in the position of equilibrium caused by a change in temperature in a reaction. 2.4.2 Changing pressure The influence of pressure The effect of changes in pressure on a system at equilibrium is only important for reactions which involve gas molecules and have a volume change involved in the chemical reaction. The equilibrium between dinitrogen tetroxide and nitrogen dioxide involves a volume change and is a suitable reaction to study. Because N2 O4 is colourless and NO2 is brown, the colour of the equilibrium mixture can give an indication of the equilibrium position. © H ERIOT-WATT U NIVERSITY 63 64 TOPIC 2. CHEMICAL EQUILIBRIA Equilibrium and pressure Go online The on-line version of this activity contains a simulation experiment in which the effect of pressure changes on the position of equilibrium can be investigated. If you do not have access to the on-line version, look at this diagram before answering the questions. Consider the effect of increased pressure. Q18: Has the total number of molecules present increased or decreased when the pressure increased? .......................................... Q19: Has the volume of gas present at the higher pressure increased or decreased? .......................................... Q20: Has the equilibrium position shifted to the right or left? .......................................... Q21: Explain how this could be predicted by Le Chatelier's principle. .......................................... Now consider the effect of decreased pressure. Q22: Use Le Chatelier's principle to explain fully the observations made. Write an answer before consulting the back of the text. .......................................... .......................................... Key point Le Chatelier's principle can be used to predict changes in the position of equilibrium in a reaction involving gas molecules due to pressure changes. © H ERIOT-WATT U NIVERSITY TOPIC 2. CHEMICAL EQUILIBRIA 2.4.3 65 The influence of concentration The equilibrium reaction between nitrogen and hydrogen as reactants, and ammonia as the product, is an extremely important industrial process. The Haber process (see below) is aimed at manufacturing ammonia with the highest possible yield. Before starting the next activity, consider Le Chatelier's principle in relation to the concentrations of reactants or products in this system. Key point Le Chatelier's principle If the conditions of a chemical system at equilibrium are changed, the system responds by minimising the effect of the changes. • Try to predict what will happen to the position of equilibrium if the concentration of a reactant is increased. • Try to predict what will happen to the position of equilibrium if the concentration of a product is increased. • What would be the best strategy to yield the highest percentage of ammonia? Changing concentration The on-line version of this activity contains graphical simulations of changes in the position of equilibrium which occur when the concentration of reactant or product are altered in the Haber process. Look at these graphs before answering the questions. Note: The concentration of substances, in mol -1 , can be represented by square brackets around the formula. Thus [H 2 ] represents "the concentration of hydrogen". The graphs show the basic equilibrium position (left) and the situation as the concentration of one reactant is increased (right). © H ERIOT-WATT U NIVERSITY Go online 66 TOPIC 2. CHEMICAL EQUILIBRIA Haber process graphs Q23: Which reactant has been added to affect the equilibrium? .......................................... Q24: In response to this, has the ammonia concentration increased or decreased? .......................................... Q25: Has the equilibrium position shifted left or right? .......................................... Q26: Explain how this could be predicted by Le Chatelier's principle. .......................................... Q27: Use Le Chatelier's principle to explain the shape of the line tracing the hydrogen concentration. .......................................... In summary, Le Chatelier's principle predicts that high concentrations of both nitrogen and hydrogen assist in shifting the position of equilibrium to the side which increases © H ERIOT-WATT U NIVERSITY TOPIC 2. CHEMICAL EQUILIBRIA ammonia yield. Constant removal of the ammonia produced also encourages the further production of the product. .......................................... 2.4.4 The influence of catalysts Catalysts are very widely used in industry to speed up chemical reactions. Without them modern chemical plants would not be able exist. Although they affect the rate of reaction, they have no effect on the equilibrium position, since they affect the rates of both the forward and reverse reactions similarly. Key point Changes in concentration, pressure and temperature can alter the position of equilibrium. Le Chatelier's principle can be used to predict changes in the position of equilibrium in a reaction. A catalyst does not alter this position, merely changes the rate at which it is attained. 2.5 The Haber process Modern agriculture relies heavily on industrially manufactured fertilisers, particularly nitrates. The Haber process produces ammonia (see below) which is a key chemical for nitrate production and is a good example of how chemists can adjust the position of equilibrium in an industrial process to provide a cost-effective yield. © H ERIOT-WATT U NIVERSITY 67 68 TOPIC 2. CHEMICAL EQUILIBRIA 2.5.1 Adjusting the concentration Changing concentration of ammonia Go online Investigate the effect that adjusting the concentration of ammonia has on the position of equilibrium by examining the graphs obtained when you "increase ammonia" or "decrease ammonia" as shown below. .......................................... Q28: Changing the concentration of ammonia will affect the position of equilibrium. Does shifting the position of equilibrium towards the product side occur if the concentration of ammonia is increased or decreased? .......................................... Q29: Explain how this could be predicted by Le Chatelier's principle. .......................................... In the Haber process, industrial chemists cool the reaction mixture and the ammonia produced condenses to a liquid form. The equilibrium responds by shifting to the right and producing more ammonia. The liquid ammonia formed is easily run off into a © H ERIOT-WATT U NIVERSITY TOPIC 2. CHEMICAL EQUILIBRIA 69 storage tank and the nitrogen and hydrogen gases are recycled through the process so there is almost no wastage. 2.5.2 Temperature in the Haber process Temperature and pressure in the Haber process The on-line version of this activity contains graphs which show how pressure and temperature changes affect the yield of ammonia in the Haber process. If you do not have access to the on-line version, look at these graphs before answering the questions. Look at the graph showing 50 atmospheres pressure. Pressure and Temperature graphs .......................................... Q30: At 50 atmospheres pressure, is the yield of ammonia highest at high or low temperature? .......................................... Q31: Explain how this could be predicted by application of Le Chatelier's principle. .......................................... Q32: In the industrial process the temperature used is actually about 400 ◦ C. Which of these could be the yield at 400 ◦ C and 50 atmospheres? a) Less than 10% ammonia b) Between 10% and 50% © H ERIOT-WATT U NIVERSITY Go online 70 TOPIC 2. CHEMICAL EQUILIBRIA c) Between 50% and 100% d) More than 100% .......................................... Q33: If the Haber process was carried out at 50 atmospheres and room temperature (25◦ C) the yield would be about 90% ammonia. In the industrial process the temperature used is actually about 400 ◦ C. Explain why the higher temperature is used even though it gives a lesser yield. (Hint: think about reaction rates). .......................................... 2.5.3 Pressure in the Haber process Temperature and pressure in the Haber process Go online The on-line version of this activity contains graphs which show how pressure and temperature changes affect the yield of ammonia in the Haber process. If you do not have access to the on-line version, look at these graphs before answering the questions. Look at the graphs showing the yield of ammonia at 100, 200 and 400 atmospheres. Pressure and Temperature graphs .......................................... Q34: Is the yield of ammonia highest at high or low pressure? .......................................... Q35: Explain how this could be predicted by application of Le Chatelier's principle. .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 2. CHEMICAL EQUILIBRIA Q36: In the industrial process the pressure used is actually about 200 atmospheres. Explain why the lower pressure is used even though it gives a lesser yield. (Hint: think about cost-effectiveness.) .......................................... Key point Pressure, temperature and catalyst can be controlled in the Haber process in order to maximise a safe, cost-effective yield of ammonia. The operating conditions in the Haber process, although easily predicted using Le Chatelier's principle, are a series of compromises which have to take into account building, maintenance, energy and labour costs, safety, catalyst activity, reaction rate and yield. The industrial process has been developed to allow the chemical industry to function in the most cost-effective way. 2.5.4 Catalysts Key point The effects of pressure and temperature, use of catalyst, removal of product and recycling of unreacted gases can be considered in relation to the conditions actually applied in the Haber process. The catalyst used in the Haber process speeds up the rate of both forward and reverse reaction and reduces the time required for equilibrium to be established. The finely divided iron used as a catalyst in the reaction only operates between certain temperatures, as illustrated in the graphs. The efficiency of the catalyst is increased by the addition of small amounts of potassium oxide or aluminium oxide which act as promoters. © H ERIOT-WATT U NIVERSITY 71 72 TOPIC 2. CHEMICAL EQUILIBRIA Summary of the Haber process Go online Q37: Copy this paragraph or photocopy the page and fill in the appropriate word from the word bank. .......................................... 2.6 Summary Summary Reversible reactions • Many reactions are reversible, so products may be in equilibrium with reactants. Equilibrium • At equilibrium, the concentrations of reactants and products remain constant, but are rarely equal. • This may result in costly reactants failing to be completely converted into products. • The same equilibrium position is reached irrespective of whether starting from reactants or products. • In a closed system, reversible reactions attain a state of dynamic equilibrium when the rates of forward and reverse reactions are equal. Altering position of equilibrium • Le Chatelier's principle states that “If the conditions of a chemical system at equilibrium are changed, the system responds by minimising the effect of the changes.” © H ERIOT-WATT U NIVERSITY TOPIC 2. CHEMICAL EQUILIBRIA Summary continued • Le Chatelier's principle can be used to predict changes in the position of equilibrium caused by a change in temperature in a reaction. • Le Chatelier's principle can be used to predict changes in the position of equilibrium in a reaction involving gas molecules due to pressure changes. • Le Chatelier's principle can be used to predict changes in the position of equilibrium in a reaction when concentration of reactants or products are altered. • Changes in concentration, pressure and temperature can alter the position of equilibrium. Le Chatelier's principle can be used to predict changes in the position of equilibrium in a reaction. • A catalyst does not alter this position, merely changes the rate at which it is attained. The Haber process 2.7 • • To maximise profits, chemists employ strategies to move the position of equilibrium in favour of products. • Pressure, temperature and catalyst can be controlled in the Haber process in order to maximise a safe, cost-effective yield of ammonia. • The effects of pressure and temperature, use of catalyst, removal of product and recycling of unreacted gases can be considered in relation to the conditions actually applied in the Haber process. Resources Higher Chemistry for CfE: J Anderson, E Allan and J Harris, Hodder Gibson, ISBN 978-1444167528 © H ERIOT-WATT U NIVERSITY 73 74 TOPIC 2. CHEMICAL EQUILIBRIA 2.8 End of topic test End of topic 2 test Go online This end of topic test is available online. If you do not have access to the internet, here is a paper version. Q38: Chemical reactions are in a state of dynamic equilibrium only when the: A) concentrations of reactants and products are equal. B) rate of forward reaction equals the rate of reverse reaction. C) reaction involves no enthalpy change. D) activation energy of the forward and reverse reactions are equal. .......................................... Q39: At equilibrium, the concentrations of reactants and products always: A) become zero. B) reach their highest. C) remain constant. D) become equal. .......................................... Q40: A two way arrow is used in an equation to show that the reaction: A) is completely finished. B) is a reversible reaction. C) has equal concentrations of reactants and products. D) is changing direction. .......................................... Q41: In an industrial process, ethene and steam react together as follows. C2 H4 (g) + H2 O(g) C2 H5 OH(g) The reaction is exothermic. Which set of conditions would give the best yield of ethanol at equilibrium? A) Low temperature, high pressure B) High temperature, high pressure C) High temperature, low pressure D) Low temperature, low pressure © H ERIOT-WATT U NIVERSITY TOPIC 2. CHEMICAL EQUILIBRIA .......................................... The questions below refer to this equilibrium reaction. 2SO2 (g) + O2 (g) 2SO3 (g) The reaction is exothermic. Q42: Increasing the temperature would cause: A) no change in the position of equilibrium. B) both reactant gases to decrease concentration. C) the proportion of sulfur trioxide to decrease. D) the position of equilibrium to shift to the right. .......................................... Q43: Decreasing the pressure would cause: A) the position of equilibrium to shift to the right. B) no change in the position of equilibrium. C) the proportion of sulfur trioxide to increase. D) both reactant gases to increase concentration. .......................................... Q44: Using a catalyst would cause: A) no change in the position of equilibrium. B) the proportion of sulfur trioxide to decrease. C) the position of equilibrium to shift to the right. D) both reactant gases to increase concentration. .......................................... © H ERIOT-WATT U NIVERSITY 75 76 TOPIC 2. CHEMICAL EQUILIBRIA Chlorine gas dissolves in water, giving the following equilibrium: Cl2 (aq) + H2 O(l) 2H+ (aq) + ClO- (aq) + Cl- (aq) Q45: Identify the compound which, if added to the equilibrium mixture, would shift the position of equilibrium to the left. A) KCl(s) B) KOH(s) C) Na2 SO4 (s) D) AgNO3 (s) E) KF(s) F) NaNO3 (s) .......................................... Q46: Identify the compounds which, if added to the equilibrium mixture, would shift the position of equilibrium to the right. A) KCl(s) B) KOH(s) C) Na2 SO4 (s) D) AgNO3 (s) E) KF(s) F) NaNO3 (s) .......................................... Q47: Identify the statements which can be applied to the role of a catalyst in a reversible reaction. A) It decreases the time required for equilibrium to be established. B) It alters the equilibrium position. C) It lowers the activation energy of the reverse reaction. D) It decreases the enthalpy change for the reaction. E) It increases the rate of the forward reaction more than the reverse reaction. .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 2. CHEMICAL EQUILIBRIA This equilibrium shows the relationship between dinitrogen tetroxide and nitrogen dioxide. N2 O4 (g) 2NO2 (g) Q48: The amount of nitrogen dioxide formed by dissociation of dinitrogen tetroxide increases as temperature rises. The dissociation of dinitrogen tetroxide is: .......................................... Q49: How would the equilibrium concentration of nitrogen dioxide be affected by a decrease in pressure? .......................................... The graph shows how the percentage of ammonia in the gas mixture at equilibrium varies with pressure at different temperatures. Q50: At what temperature would a pressure of 100 atmospheres give a yield of 20%? .......................................... Q51: In the industrial process the percentage yield of ammonia never rises above 15%. Although the yield is low, the process is still profitable. What is done to the excess nitrogen and hydrogen which allows the process to be profitable? .......................................... .......................................... © H ERIOT-WATT U NIVERSITY 77 78 TOPIC 2. CHEMICAL EQUILIBRIA © H ERIOT-WATT U NIVERSITY 79 Topic 3 Chemical energy Contents 3.1 Prior knowledge . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 83 3.2 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.3 Potential energy diagrams . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 84 86 3.3.1 Enthalpy changes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.3.2 What produces this energy change? . . . . . . . . . . . . . . . . . . . . 87 90 3.3.3 The thermochemical equation . . . . . . . . . . . . . . . . . . . . . . . 3.4 Enthalpy changes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 92 95 3.4.1 Enthalpy of combustion . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.4.2 Enthalpy of solution . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 97 99 3.4.3 Enthalpy of neutralisation . . . . . . . . . . . . . . . . . . . . . . . . . . 3.5 Introduction to Hess's law . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 102 106 3.5.1 Hess's law . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.5.2 Verification of Hess's law . . . . . . . . . . . . . . . . . . . . . . . . . . 108 108 3.5.3 Applications of Hess's law . . . . . . . . . . . . . . . . . . . . . . . . . . 3.6 Bond enthalpies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 112 116 3.7 Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 118 3.8 Resources . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.9 End of topic test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 120 121 80 TOPIC 3. CHEMICAL ENERGY Prerequisite knowledge Before you begin this topic, you should know: • the gram formula mass is defined as the mass of one mole of a substance (National 5, Unit 1); • using the chemical formula of any substance the gram formula mass can be calculated using relative formula masses of its constituent elements (National 5, Unit 1); • the concentration of solutions in moles per litre (National 5, Unit 1); • calculations to determine the concentration and volume and the mass of a substance through the number of moles present (National 5, Unit 1); • define types of reaction (eg. neutralisation, combustion) (National 5); • alkanes and alcohols can be used as fuels (National 5, Unit 2); • combustion reactions are exothermic reactions (National 5, Unit 2); • the opposite of this is an endothermic reaction (National 5, Unit 2); • when a substance is combusted the reaction can be represented using a balanced formulae equation (National 5, Unit 2); • the quantities of reactants and products in these reactions can be calculated (National 5, Unit 2); • different fuels provide different quantities of energy and this can be measured experimentally and calculated using Eh = cmΔT (National 5, Unit 2). © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY Learning objectives At the end of this topic, you should be able to state that: Chemical energy • For industrial processes, it is essential that chemists can predict the quantity of heat energy taken in or given out. • If reactions are endothermic, costs will be incurred in supplying heat energy in order to maintain the reaction rate. • If reactions are exothermic, the heat produced may need to be removed to prevent the temperature rising. Enthalpy • Chemical energy is also known as enthalpy. • The change in chemical energy associated with chemical reactions can be measured. The specific heat capacity, mass and temperature can be used to calculate the enthalpy change for a reaction. • The enthalpy of combustion of a substance is the enthalpy change when one mole of the substance burns completely in oxygen. • These values can often be directly measured using a calorimeter and values for common compounds are available from data books and online databases for use in Hess’s law calculations. Hess’s law • Hess’s law states that the enthalpy change for a chemical reaction is independent of the route taken. • Enthalpy changes can be calculated by applying Hess’s law. Bond enthalpies • For a diatomic molecule, XY, the molar bond enthalpy is the energy required to break one mole of XY bonds. • Mean molar bond enthalpies are average values which are quoted for bonds which occur in different molecular environments. • Bond enthalpies can be used to estimate the enthalpy change occurring for a gas phase reaction by calculating the energy required to break bonds in the reactants and the energy released when new bonds are formed in the products. © H ERIOT-WATT U NIVERSITY 81 82 TOPIC 3. CHEMICAL ENERGY Learning objective At the end of this topic, you should be able to state that: Chemical energy • For industrial processes, it is essential that chemists can predict the quantity of heat energy taken in or given out. • If reactions are endothermic, costs will be incurred in supplying heat energy in order to maintain the reaction rate. • If reactions are exothermic, the heat produced may need to be removed to prevent the temperature rising. Enthalpy • Chemical energy is also known as enthalpy. • The change in chemical energy associated with chemical reactions can be measured. The specific heat capacity, mass and temperature can be used to calculate the enthalpy change for a reaction. • The enthalpy of combustion of a substance is the enthalpy change when one mole of the substance burns completely in oxygen. • These values can often be directly measured using a calorimeter and values for common compounds are available from data books and online databases for use in Hess’s law calculations. Hess’s law • Hess’s law states that the enthalpy change for a chemical reaction is independent of the route taken. • Enthalpy changes can be calculated by applying Hess’s law. Bond enthalpies • For a diatomic molecule, XY, the molar bond enthalpy is the energy required to break one mole of XY bonds. • Mean molar bond enthalpies are average values which are quoted for bonds which occur in different molecular environments. • Bond enthalpies can be used to estimate the enthalpy change occurring for a gas phase reaction by calculating the energy required to break bonds in the reactants and the energy released when new bonds are formed in the products. © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY 3.1 83 Prior knowledge Test your prior knowledge Q1: In which of the following reactions is oxygen used up? a) b) c) d) Go online Combustion Neutralisation Addition Polymerisation .......................................... Q2: Which of the following is a correctly balanced equation? a) b) c) d) Li2 CO3 (s) + HCl(aq) → LiCl(aq) + CO2 (g) + H2 O(l) Li2 CO3 (s) + HCl(aq) → 2LiCl(aq) + CO2 (g) + H2 O(l) Li2 CO3 (s) + 2HCl(aq) → 2LiCl(aq) + CO2 (g) + H2 O(l) Li2 CO3 (s) + HCl(aq) → 2LiCl(aq) + 2CO2 (g) + 2H2 O(l) .......................................... Q3: What name is given to any chemical reaction which releases energy? a) b) c) d) Exothermic Endothermic Combustion Neutralisation .......................................... Q4: The student recorded the following data. Mass of alkane burned 1g Volume of water Initial temperature of water 200 cm3 15◦ C Final temperature of water 55◦ C Specific heat capacity of water 4.18 kJ kg-1 ◦ C-1 How much energy was released, in kJ? a) b) c) d) 33440 33.44 167.2 45.98 .......................................... © H ERIOT-WATT U NIVERSITY 84 TOPIC 3. CHEMICAL ENERGY Q5: The reaction above is an example of: a) b) c) d) hydration. addition. condensation. saturation. .......................................... 3.2 Introduction Much of the energy that we use in everyday activities is derived from the chemical potential energy available from substances we call "fuels". Our cars are powered by the energy released when petrol burns, many houses are heated by burning natural gas (methane), electricity is mainly produced by burning coal or natural gas, and our bodies are powered by using energy-rich foods like sugars and fats. The energy stored in these sources (chemical potential energy) can be released when these substances undergo chemical reactions, often involving oxidation. The energy can be released in various forms such as heat, light, sound, electricity. A chemical reaction producing heat, light and sound energy. © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY In fact many chemical reactions release heat to the surroundings as they proceed. Examples: Metals reacting with acids; acids neutralising alkalis. The "Thermite" reaction generates enough heat to melt iron, and is used to weld rails together. Reactions which release heat to the surroundings are called exothermic reactions. Some reactions, however, absorb heat from the surroundings as they proceed. Examples: Dissolving ammonium nitrate in water; solid ammonium thiocyanate and barium hydroxide reacting. The "cool packs" used for sports injuries contain chemicals which cool down the pack when they react. These types of reaction are called endothermic reactions. Key point Exothermic changes cause heat to be released to the surroundings; endothermic changes cause absorption of heat from the surroundings. The importance to industry Any changes in the heat produced or absorbed by the reactions a in a chemical production process have to be accounted for. Exothermic reactions may require cooling; endothermic ones might need a supply of heat. Part of the energy economics of a process plant is to try to use heat removed from one reaction to heat another. It will cost if heat is wasted directly to the atmosphere, or if fuels have to be purchased specially. © H ERIOT-WATT U NIVERSITY 85 86 TOPIC 3. CHEMICAL ENERGY 3.3 Potential energy diagrams Consider the exothermic reaction between zinc and sulfuric acid. After the reaction, the energy stored in the products is less than the energy originally stored in the reactants - that is, some of the chemical potential energy stored in reactants has been transferred to the surroundings. This transferred heat energy is defined as the enthalpy change for this reaction. Strictly, the change in energy which occurs during a chemical reaction consists of two components: heat and work. We live on the surface of the Earth, at the bottom of a 'sea' of atmosphere which exerts pressure. In the reaction above, the gaseous hydrogen produced will have to 'work' to displace the atmosphere. This will require use of some energy, so the heat energy released to the surroundings will be less than the total energy change. In this reaction, for 1 mole of zinc, the heat (enthalpy) change is 152.4 kJ and the work against the atmosphere 2.5 kJ, the overall energy change being 154.9 kJ. Chemists often carry out reactions at constant pressure, hence the use of 'enthalpy change', the heat change at constant pressure. Let's consider another familiar exothermic reaction, the burning (oxidation) of methane in a flame. You see this every time you use natural gas. These changes are often shown as a potential energy diagram. An example is shown in Figure 3.1 for methane oxidation. © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY Figure 3.1: Methane oxidation .......................................... This diagram shows the potential energy of the reactants, at the start of the reaction. It also shows the potential energy of the products, at the end of the reaction. The arrow shows the energy given to the surroundings. 3.3.1 Enthalpy changes The easiest measurement to make is the enthalpy change, ΔH, when the reaction occurs. This is defined as the difference in enthalpy of the products and reactants by the equation below. The units of energy and enthalpy are joules (J). ΔH is negative for an exothermic reaction. The energy of the products is less than that of the reactants. © H ERIOT-WATT U NIVERSITY 87 88 TOPIC 3. CHEMICAL ENERGY The potential energy diagram for an endothermic reaction, the decomposition of calcium carbonate. This is shown below. In this case the energy of the products is greater than that of the reactants. Q6: So what will happen to the temperature of the surroundings? a) It will increase. b) It will not change. c) It will decrease. .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY Q7: What will the value of ΔH be? a) Negative b) Zero c) Positive .......................................... This is shown below. In order to distinguish endothermic and exothermic reactions the + and - sign are always used when giving ΔH values. Q8: The equation for the "water gas" reaction is: Figure 3.2: Water gas reaction .......................................... Note that this equation states that the carbon is in the solid state(s), and the other materials are gases (g). This reaction absorbs energy from its surroundings. What type of reaction is this? a) b) c) d) Fast Exothermic Explosive Endothermic .......................................... © H ERIOT-WATT U NIVERSITY 89 90 TOPIC 3. CHEMICAL ENERGY What will be the sign of ΔH? Q9: a) b) c) d) Positive. Has no sign. Negative. Insufficient information to know. .......................................... Q10: Draw a potential energy diagram for this reaction, and compare it with the answer at the back of the book. .......................................... But how much energy is involved in this reaction? In the case of the water gas reaction (Figure 3.2) 121 kJ is absorbed for each mole of carbon and steam used. In other words ΔH = +121 kJ mol-1 . The full potential energy diagram for the water gas reaction is shown below. Key point 3.3.2 • A potential energy diagram can be used to show the energy changes for a reaction. • The enthalpy change is the energy difference between products and reactants. • The enthalpy change can be calculated from a potential energy diagram. • The enthalpy change has a negative value for exothermic reactions and a positive value for endothermic reactions. What produces this energy change? The chemical energy in molecules is stored in the bonds. When a reaction occurs the bonds in the reactant molecules are broken and the atoms rearrange to form new bonds with different energies in the products. For example, think of making ammonia from nitrogen and hydrogen. © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY The balanced chemical equation is: You can think of this reaction as first breaking the bonds in the nitrogen and hydrogen molecules. This stage requires an input of energy. Then these atoms rearrange and combine to form new N - H bonds in ammonia. This stage releases energy. © H ERIOT-WATT U NIVERSITY 91 92 TOPIC 3. CHEMICAL ENERGY The enthalpy change will be given by the total energy in new bonds minus the energy present in the original bonds. It is important to remember that reactions do not normally proceed by such a route, but the enthalpy change can be calculated as if they did. 3.3.3 The thermochemical equation It is usual to express this information in a thermochemical equation, such as that for the water gas reaction below (Figure 3.3). Figure 3.3: Thermochemical equation for the water gas reaction .......................................... Three points should be noted. 1. The enthalpy value quoted in the balanced equation is measured in kilojoules per mole (kJ mol-1 ). Look at Figure 3.3 again. The 121 kJ of energy is absorbed when one mole of solid carbon and one mole of steam react; two moles of each reacting would absorb 242 kJ, and so on. (This makes sense when you think that burning 2 kg of carbon would give out twice the energy from burning 1 kg.) 2. The equation always contains the state symbols of reactants and products. The enthalpy of reactions will change if the states are different. Look carefully at these two equations for the reaction of hydrogen and oxygen H2(g) + ½O2(g) H2O(g) ΔH = -242 kJ mol-1 (N.B. the equation has been written for forming 1 mole of water. The " 1 /2 " before the oxygen means 1 /2 mole of reactant not 1 /2 a molecule of oxygen.) but © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY The extra enthalpy in the second case is due to the heat released when the water vapour condenses to liquid water. 3. Since the enthalpy change is independent of the route of the reaction, the enthalpy change for the reverse reaction will be equal in value, but of opposite sign. For the reaction below: Q11: So the enthalpy change for the reverse reaction: CO2 (g) → CO(g) + 1 /2 O2 (g) will be? a) b) c) d) ΔH = -566 kJ mol-1 ΔH = -283 kJ mol-1 ΔH = 0 kJ mol-1 ΔH = +283 kJ mol-1 .......................................... Q12: The equation for calculating the enthalpy change (ΔH) for a reaction is: a) b) c) d) ΔH = Hproducts + Hreactants ΔH = Hproducts - Hreactants ΔH = Hreactants + Hproducts ΔH = Hreactants - Hproducts .......................................... © H ERIOT-WATT U NIVERSITY 93 94 TOPIC 3. CHEMICAL ENERGY Q13: A ΔH value of - 500 kJ mol -1 would indicate that the reaction is: a) b) c) d) Fast Reversible Exothermic Endothermic .......................................... Q14: The potential energy diagram for the combustion of one mole of ethene is: What is the enthalpy change for this reaction? a) b) c) d) ΔH = +49 kJ mol-1 ΔH = -1313 kJ mol-1 ΔH = -1362 kJ mol-1 ΔH = -1411 kJ mol-1 .......................................... Q15: The equation for the combustion of ethanol is: How many kJ of energy will be released to the surroundings if 5 moles of ethanol are burned? .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY Q16: Given that: What would be the enthalpy change for the reaction below? (Hint: don't forget the sign.) .......................................... Q17: The Ostwald process for producing nitric acid involves the oxidation of ammonia (NH3 ) to form nitric oxide (NO) and water. Write a balanced equation for this reaction. .......................................... Q18: Given that the enthalpy of the reactants (as written in the balanced equation) is -184 kJ and the enthalpy of the products is -1090 kJ, calculate the enthalpy change for this reaction in kJ. Remember the sign. .......................................... Q19: What would be the enthalpy change per mole of ammonia oxidised? Give your answer in kJ mol-1 to 1 decimal place. .......................................... Q20: When 102 g of ammonia is oxidised, how many kJ of heat have to be removed to maintain a constant temperature? .......................................... 3.4 Enthalpy changes All chemical (and many physical) processes are associated with a particular enthalpy change, but these are often difficult to measure. Three particular enthalpy changes which can be measured in the laboratory are: • enthalpy of combustion; • enthalpy of solution, and; • enthalpy of neutralisation. The methods of obtaining these values will be considered as well as their importance. © H ERIOT-WATT U NIVERSITY 95 96 TOPIC 3. CHEMICAL ENERGY The change in temperature during a reaction allows the heat energy change and ΔH to be measured. To obtain the most accurate result we also want to limit losses of heat from our system, so that some form of insulation is normally employed to minimise these losses. All the methods depend on measuring the temperature change of a known mass of water (or dilute solution) during the course of a reaction. The temperature change is related to the heat energy by using the equation shown in Figure 3.4 Figure 3.4: Heat transfer equation .......................................... The heat energy transferred is in kJ, c is the specific heat capacity of water. The specific heat capacity relates the energy change in a liquid to the change in temperature. For water it has a value of 4.18 kJ kg -1 ◦ C-1 . In other words, when 1 kg of water absorbs 4.18 kJ of heat its temperature will rise by 1◦ C. m is the mass of water, or solution (in kg), and ΔT is the temperature change (in ◦ C). The use of this equation will be illustrated in the three particular cases described in the next sections. © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY 3.4.1 97 Enthalpy of combustion The enthalpy of combustion is defined as the enthalpy change that occurs when 1 mole of a substance is burned completely in oxygen. It is often called ΔH c . To determine the heat given out from a measured quantity of a liquid fuel, say, ethanol, the fuel is burned and the heat produced warms a known mass (or volume) of water. This experiment and the calculation is described in the next activity. Determination of the enthalpy of combustion of an alcohol A interactive description of this experiment is available on-line. The equipment used is shown below. Enthalpy of combustion equipment The spirit burner containing ethanol is weighed at the beginning and end of the experiment to obtain the mass of ethanol used. Before the experiment we should know the mass (or volume) of the water to be heated. The temperature of the water in the can is measured at the start and finish, and the temperature change noted. We now have all the information to construct a table of data to determine the enthalpy of combustion of ethanol. An example set of data is shown in the following table. © H ERIOT-WATT U NIVERSITY Go online 98 TOPIC 3. CHEMICAL ENERGY Readings taken Figure obtained Value Mass of burner at start & end Mass of ethanol burned 0.368 g Volume of water heated Mass of water (m) 0.200 kg Temperature of water at start & end Temperature rise (ΔT) +12.0 o C - Specific heat of water (c) 4.18 kJ o C-1 kg-1 Substituting these data into the equation in Figure 3.4 Energy transferred = c m ΔT = 4.18 kJ kg - 1 o C - 1 × 0.200 kg × ( + 12.0 o C) = 4.18 × 0.200 × 12.0 kJ (kg - 1 o C - 1 kg o C cancel out) = 10.032 kJ So 10.032 kJ of energy are released when 0.368 g of ethanol are burned. We need to find how many kJ are released when 1 mole of ethanol burns. Calculate the gram formula mass of ethanol. Then work out the energy released when 1 mol of ethanol burns. 0.368 g of ethanol releases 10.032 kJ 0.368/46 mols of ethanol releases 10.032 kJ 0.008 mols of ethanol releases 10.032 kJ 1 mol of ethanol releases 10.032/0.008 kJ 1 mol of ethanol releases 1254 kJ Remember that the heat has been measured in the water, i.e. energy is released to the surroundings. The combustion of ethanol is exothermic, and the enthalpy change for the reaction will be negative. The enthalpy of combustion of ethanol (ΔH c ) is -1254 kJ mol-1 . © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY 99 The value from the data booklet is -1367 kJ mol -1 . This experimental value is lower than the quoted one. Q21: Can you think of some reasons for this? .......................................... Enthalpy of combustion questions Q22: Use the data booklet to find the enthalpy of combustion of propane and butane. If you had a mole of each fuel, which would give you most heat energy? a) Propane b) Butane .......................................... Q23: You are heating a pan containing 1.0 kg of water using a butane burner. If 0.1 mol of butane is used, what would be the temperature rise of the water? Give your answer to 1 decimal place. .......................................... Q24: Your friend is using a methanol (CH 3 OH) burner to heat her water. She finds that burning 6.4g of methanol raises the temperature of 0.50 kg of water by 50 ◦ C. From these data what is the enthalpy of combustion of methanol? Give your answer in kJ mol -1 to 1 decimal place, and remember the sign. .......................................... 3.4.2 Enthalpy of solution The enthalpy of solution is defined as the energy change (in kJ) when 1 mole of the substance dissolves in water. To determine the enthalpy of solution of a substance in water, a known weight of it is dissolved in a known volume of water. The temperature change is noted when the substance has dissolved. The experiment is carried out under conditions where the system (substance plus water) is as thermally insulated from the surroundings as possible. For practical purposes polystyrene cups are very useful - they have good insulation and themselves have a very low specific heat capacity. The enthalpy change in this case results from the difference between the enthalpy input required to disrupt the crystal lattice (see ionic lattices) and the enthalpy obtained when the ions are hydrated. Enthalpies of solution can be +ve or -ve, unlike ΔH c which is always -ve. An experiment to determine the enthalpy of solution of ammonium nitrate is described in the next activity. © H ERIOT-WATT U NIVERSITY Go online 100 TOPIC 3. CHEMICAL ENERGY An experiment to determine the enthalpy of solution of ammonium nitrate Go online Q25: In order to use the equation Energy transferred = c m ΔT what information do you need at the start of the experiment? .......................................... Q26: During the experiment, the temperature drops to a minimum, then slowly rises again. Why do you think the temperature behaves like this? .......................................... The lowest temperature reached was 20.4 ◦ C. Q27: If the initial temperature was 22.0 ◦ C, what is the value of ΔT? .......................................... Calculation of the enthalpy of solution of ammonium nitrate, using the experimental data above The balanced equation for the reaction is NH4 NO3 (s) → NH4 + (aq) + NO3 - (aq) Put the experimental data into a table: © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY 101 Readings taken Figure obtained Value Mass of ammonium nitrate - 1.00vg Volume of water Mass of water 0.050 kg Temperature of water at start Temperature of solution at end Temperature change - 1.6 ◦ C - Specific heat of water 4.18 kJ kg-1 ◦ C-1 N.B. Temperature change is -1.6 ◦ C (the temperature dropped, so ΔT is negative). Once again, we use the equation in Figure 3.4, making the assumption that the specific heat of the ammonium nitrate solution is the same as that of water. Substituting into the equation gives, Energy transferred = c m ΔT = 4.18 kJ kg - 1 o C - 1 × 0.050 kg × ( - 1.6 o C) = 4.18 × 0.050 × ( - 1.6) kJ = - 0.334 kJ 0.334 kJ of energy was transferred from the surroundings (negative value) when 1g of ammonium nitrate was dissolved in water. The gram formula mass of NH 4 NO3 is Calculate the number of kJ of energy absorbed when 1 mol of ammonium nitrate dissolves. 1.00g of ammonium nitrate absorbed 0.334 kJ of energy 1.00/80 mol of ammonium nitrate absorbed 0.334 kJ of energy 0.0125 mol of ammonium nitrate absorbed 0.334 kJ of energy 1 mol of ammonium nitrate absorbed 0.334/0.0125 kJ of energy 1 mol of ammonium nitrate absorbed 26.7 kJ of energy © H ERIOT-WATT U NIVERSITY 102 TOPIC 3. CHEMICAL ENERGY The enthalpy of solution of ammonium nitrate is +26.7 kJ mol -1 . NH4 NO3 (s) → NH4 + (aq) + NO3 - (aq) ΔH = +26.7 kJ mol-1 (Remember a final check, heat was absorbed as the temperature dropped, the reaction is endothermic, ΔH will be +ve.) .......................................... Questions on enthalpy of solution Q28: When 1.20 g of sodium hydroxide is dissolved in 60 cm 3 of water the temperature rises from 18.4◦ C initially to 23.2◦ C in the final solution. What is the enthalpy of solution of sodium hydroxide in kJ mol-1 , to 1 decimal place? .......................................... Q29: Chemical heat packs, used to prevent hypothermia, release heat when calcium chloride dissolves in water according to the equation: How many kJ, to 3 significant figures, of energy will be obtained from a pack containing 500 g of calcium chloride? .......................................... Key point Enthalpy of solution is the energy change when one mole of a substance dissolves in water. The units are kJ mol-1 . 3.4.3 Enthalpy of neutralisation The enthalpy of neutralisation of an acid is defined as the energy change (in kJ) when it is neutralised to form 1 mole of water. Enthalpy changes for this reaction can be obtained by using polystyrene cups to reduce heat loss to a minimum when a known volume of alkali is added to an equivalent amount of acid and the temperature change noted. The equipment to determine the enthalpy of neutralisation of hydrochloric acid is shown below. © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY 103 The results for two different combinations of acid and alkali are described. Calculation of the enthalpy of neutralisation of hydrochloric acid with sodium hydroxide. The temperatures taken were: Temperature of the acid initially 20.5 ◦ C, Temperature of the alkali initially 21.0 ◦ C, Temperature of the final solution 27.5 ◦ C. So, summarising the experimental data in a table. Readings taken Figure obtained Value Volume & concentration of acid Moles of acid reacting* 0.1 mol Volume & concentration of alkali Moles of alkali reacting* 0.1 mol Initial temperature of alkali Initial temperature of acid Average initial temperature** 20.75◦C Temperature of final solution Temperature change ΔT = +6.75◦ C - Specific heat of water 4.18 kJ kg-1 ◦ C-1 © H ERIOT-WATT U NIVERSITY 104 TOPIC 3. CHEMICAL ENERGY * Remember the equation Moles Volume (litres) Which will rearrange to give Molar concentration = Moles = Concentration x Volume In both these cases, the concentration is 1 mol l −1 and volume 0.1 l. So Moles = 1 x 0.1 mol (l −1 l cancel out) = 0.1 mols ** The initial temperature is calculated as the average of the temperatures of the acid and alkali solutions. (It would be nice to mix them and take the initial temperature, but they react on mixing, so the average initial temperature is obtained mathematically.) Temperature of acid is 20.5 o C Temperature of alkali is 21.0 o C So average initial temperature is 20.5 + 21.0 = 20.75 o C 2 The temperature change (ΔT) is then Final temperature of solution - average initial temperature 27.5 - 20.75 = + 6.75 o C We are going to use the equation in Figure 3.4 to calculate the heat change in the reaction. Remember that m = 0.200 kg (100 cm 3 of acid + 100 cm3 of alkali) Energy transferred = c m ΔT = 4.18 kJ kg - 1 o C - 1 × 0.200 kg × ( + 6.75 o C) = 4.18 × 0.200 × ( + 6.75) kJ (kg - 1 o C - 1 kg o C cancel out) = 5.64 kJ How many moles of water have been produced? Q30: 0.1 mol of hydrochloric acid and 0.1 mol sodium hydroxide reacted, so how many moles of water were produced? © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY .......................................... 5.64 kJ of energy were released to the surroundings when 0.1 moles of water were produced. So 56.4 kJ of energy were produced for each mole of water. Therefore ΔH for this neutralisation is -56.4 kJ mol -1 . Remember to check that you have written a negative value of ΔH for an exothermic reaction. The next questions concern the enthalpy of neutralisation of sulfuric acid by potassium hydroxide, given the following data. Sulfuric acid solution 50 cm3 of 1 mol -1 H2 SO4 Potassium hydroxide 50 cm3 of 2 mol -1 KOH Initial temperature of acid 21.2 ◦ C Initial temperature of alkali 21.6 ◦ C Final temperature after mixing 35.0 ◦ C Q31: Write a balanced equation for the reaction between sulfuric acid and potassium hydroxide. .......................................... Q32: What is the average initial temperature? .......................................... Q33: What is the temperature change (ΔT)? .......................................... Q34: What mass of solution was heated (m in kg)? .......................................... Q35: How much energy (in kJ) has been transferred to the water? Give your answer to 3 decimal places. .......................................... Q36: How many moles of water were produced when the sulfuric acid and potassium hydroxide reacted? .......................................... Q37: Calculate the enthalpy of neutralisation of sulfuric acid by potassium hydroxide, in kJ mol-1 , to 2 decimal places (don't forget the sign). .......................................... © H ERIOT-WATT U NIVERSITY 105 106 TOPIC 3. CHEMICAL ENERGY Q38: What do you notice about the enthalpy of neutralisation of hydrochloric acid by sodium hydroxide and of sulfuric acid by potassium hydroxide? .......................................... Q39: Can you think of a reason for this similarity? Hint: All four reactants are completely ionised in solution, so write ionic equations and think about the spectator ions. .......................................... Key point The enthalpy of neutralisation of an acid is the enthalpy change when the acid is neutralised to form one mole of water. Enthalpy changes can be calculated using the equation: energy transferred = c m ΔT. 3.5 Introduction to Hess's law You now know that the enthalpy change for a chemical reaction is described as the energy transferred to the surroundings during an exothermic reaction or the energy absorbed from the surroundings during an endothermic reaction. The enthalpy change is therefore the difference in chemical potential energy between the reactants and products, as shown in the potential energy diagrams (Figure 3.5). Figure 3.5: Potential energy diagrams .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY Q40: Which diagram (Figure 3.5) represents an exothermic reaction? .......................................... Q41: Which of the following is the enthalpy change in reaction A? a) b) c) d) -630 -570 +570 +630 .......................................... In the following diagram, the change in potential energy for the reaction between A 2 and B2 is shown, both in the presence, and in the absence, of a catalyst. The numbers 1- 4 are pointing at energy changes and numbers 5 and 6 at key points on each curve. Work out what each number is indicating (you might like to make a list) before revealing the answers by moving the mouse pointer over each number in turn. Potential Energy diagram for a catalysed reaction Q42: Which number is pointing at the enthalpy change for the reaction? .......................................... Q43: What is the significance of the energy changes indicated by 1 and 2? .......................................... Key point • The use of a catalyst provides an alternative route for a reaction. © H ERIOT-WATT U NIVERSITY 107 108 TOPIC 3. CHEMICAL ENERGY Key point continued 3.5.1 • The starting point and finishing point for both routes are the same and so the enthalpy change is the same for both routes. • Chemists make use of this in Hess's law. Hess's law Hess's law can be stated in many different ways. One such statement is: Key point Hess's law The enthalpy change for a chemical reaction is independent of the route taken, providing the starting point and finishing point is the same for both routes. This statement follows on from the law of Conservation of Energy - energy cannot be created or destroyed. Imagine a reaction which can take place by two different routes. B can be made directly from A (Route 1) or via C (route 2). According to Hess's law, ΔH1 = ΔH2 + ΔH3 . If this were not so, it would be possible to create energy, e.g. Suppose ΔH1 = -50 kJ but ΔH2 + ΔH3 = -60 kJ It would then be possible to make B by Route 2 and release 60 kJ of energy. B could then be converted back into A by reversing Route 1. This would use up 50 kJ of energy and there would be a net gain of 10 kJ. Each time this cycle was repeated, 10 kJ of energy would be created. This is impossible since it would contravene the law of Conservation of Energy. Consequently the enthalpy change for Route 1 (ΔH 1 ) must equal the enthalpy change for Route 2 (ΔH 2 + ΔH3 ), i.e. Hess's law must apply. 3.5.2 Verification of Hess's law Hess's law can be verified experimentally. Sodium chloride solution can be made from solid sodium hydroxide by two slightly different routes. © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY Route A directly by reacting solid sodium hydroxide with hydrochloric acid. Route B by first dissolving the solid sodium hydroxide in water and then neutralising the resultant solution with hydrochloric acid. All three steps involve reactions whose enthalpy changes are easily measured. The reactions can be carried out in polystyrene cups using accurately measured quantities. Route A For Route A, the temperature of a known volume of 1.0 mol -1 hydrochloric acid is measured before the addition of an accurately weighed sample of solid sodium hydroxide. The temperature rises and the highest temperature is recorded as the final temperature. From this information, the enthalpy change can be calculated. The preceding figure contains all the information necessary for the calculation. The following assumptions can be made: • assume there is no heat loss to the polystyrene cups or the surroundings; © H ERIOT-WATT U NIVERSITY 109 110 TOPIC 3. CHEMICAL ENERGY • assume the solutions have the same specific heat capacity as water (data booklet page 19); • convert masses to kilogram units. Q44: What relationship is used to calculate the energy released from the temperature rise? .......................................... Q45: Calculate the enthalpy change in kJ mol -1 . Write your answer on paper, setting out the calculation clearly, before displaying the worked answer. .......................................... For Route B, step 1 involves dissolving sodium hydroxide in water and measuring the temperature change as before. Figure 3.6: Route B, Step 1 .......................................... Q46: Use the Figure 3.6 diagram to obtain the required data and then calculate the enthalpy of solution of sodium hydroxide. If you are not confident about this or if you get the answer wrong, try the further questions on the page noted below. .......................................... Q47: What is the temperature rise (to one decimal place)? .......................................... Q48: What mass of water is being heated? .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY Q49: How much energy is released when 0.80 g of NaOH dissolves? .......................................... Q50: How much energy is released when 1 mole of NaOH dissolves? .......................................... Q51: What is the enthalpy of solution? .......................................... In step 2, the final solution from the first step (Figure 3.6) is cooled down and neutralised by a known volume of hydrochloric acid and the temperature change is measured. The initial temperature is obtained by measuring the temperature of each solution and taking the average. Figure 3.7: Route B, Step 2 .......................................... Q52: Use the diagram (Figure 3.7) to obtain the required data and then calculate the enthalpy of neutralisation of sodium hydroxide with hydrochloric acid. If you are not confident about this or if you get the answer wrong, try the further questions on the page noted below. .......................................... Q53: What is the temperature rise (to one decimal place)? .......................................... Q54: What mass of water is being heated? .......................................... Q55: How much energy is released when the two solutions are mixed? .......................................... © H ERIOT-WATT U NIVERSITY 111 112 TOPIC 3. CHEMICAL ENERGY Q56: How much energy is released when 1 mole of NaOH is neutralised? .......................................... Q57: What is the enthalpy of neutralisation? .......................................... Q58: Finally, calculate the total enthalpy change for Route B. .......................................... The enthalpy changes for Route A and Route B are very similar and well within experimental error and therefore Hess's law is obeyed. The assumptions made before the calculation was done are important when considering the experimental errors. 3.5.3 Applications of Hess's law Hess's law is important to chemists as they are often required to find values for enthalpy changes which cannot be measured directly because the reaction does not occur under normal conditions. It is difficult to burn carbon and form carbon monoxide only (Figure 3.8 (b)). So the value of ΔH2 for the formation of carbon monoxide would be difficult to determine by experiment. Figure 3.8 .......................................... According to Hess's law and the enthalpy cycles shown in Figure 3.8: ΔH1 = ΔH2 + ΔH3 Hess's law can be applied in a situation like this to enable a determination of the value of ΔH2 to be made. The first of the next two activities uses an enthalpy cycle diagram and the second uses an algebraic method to calculate a value of ΔH 2 . You may be directed to either or both methods of calculation. Application of Hess's law by enthalpy cycle diagram The Question Go online Calculate a value for the enthalpy change involved in the formation of one mole of © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY 113 carbon monoxide from carbon (graphite). The enthalpy of combustion of carbon to form carbon dioxide is quoted in the data booklet and the enthalpy of combustion of carbon monoxide is -283 kJ mol-1 . The on-line version of this activity contains an interactivity showing the stages in the calculation. If you do not have access to the on-line material use this energy cycle diagram of the reactions, and work through the text. Hess's law cycle for carbon monoxide formation The overall enthalpy change is independent of the route taken and values of ΔH 1 and ΔH3 are measurable. Since: ΔH1 = ΔH2 + ΔH3 ΔH1 = enthalpy of combustion (C to CO2 ) = − 394 kJ mol−1 ΔH3 = enthalpy of combustion (CO to CO2 ) = − 283 kJ mol−1 ΔH1 =ΔH2 + ΔH3 by rearranging ΔH2 =ΔH1 − ΔH3 ΔH2 = − 394 − (−283) kJ mol−1 ΔH2 = − 111 kJ mol−1 So the enthalpy change involved in formation of carbon monoxide from carbon (graphite) = -111 kJ mol -1 Notice that the enthalpy change represented by ΔH 3 had to be reversed in solving for ΔH2 . The numerical value of the enthalpy change therefore changed from -283 kJ mol-1 to +283 kJ mol-1 . In the same way, if an equation is multiplied or divided, the value of ΔH should be treated in the same way. Q59: Since: C(s) + 1 /2 O2 (g) → CO(g) ΔH = -111 kJ What would be the value (in kJ) for the enthalpy change for this reaction? 1 / C(s) + 1 / O (g) → 1 / CO(g) ΔH = ? kJ 2 4 2 2 .......................................... © H ERIOT-WATT U NIVERSITY 114 TOPIC 3. CHEMICAL ENERGY Q60: Since: C(s) + 1 /2 O2 (g) → CO(g) ΔH = -111 kJ What would be the value (in kJ) for the enthalpy change for this reaction? 2CO(g) → 2C(s) + O2 (g) ΔH = ? kJ .......................................... Key point Enthalpy cycle diagrams can be used to calculate enthalpy changes for reactions which are difficult to measure. .......................................... Application of Hess's law by algebraic calculation Go online Some Hess's law problems are best solved by use of an algebraic treatment of the balanced chemical equations. This activity sets out to solve the same problem as in the last activity, but uses the equations rather than an enthalpy cycle diagram. The Question Calculate a value for the enthalpy change involved in the formation of one mole of carbon monoxide from carbon (graphite). The enthalpy of combustion of carbon to form carbon dioxide is quoted in the data booklet and the enthalpy of combustion of carbon monoxide is -283 kJ mol-1 . The on-line version of this activity contains an activity showing the stages in the calculation. If you do not have access to the on-line material work your way through the text. The equation which represents the enthalpy change to be calculated is called the "target" equation. STEP 1 Write the 'target' equation. C(s) + 1 /2 O2 (g) → CO(g) ΔH = ? STEP 2 Write equations for all the information given to you. equation (a) C(s) + O2 (g) → CO2 (g) ΔH = -394 kJ (from data booklet) equation (b) CO(g) + 1 /2 O2 (g) → CO2 (g) ΔH = -283 kJ © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY 115 To construct the "target" equation from (a) and (b), leave equation (a) as written and reverse equation (b). Hess's Law allows you to treat the ΔH values the same way. The website animation shows the stages. STEP 3 STEP 3 continued equation (a) C(s) + O2 (g) → CO2 (g) ΔH = -394 kJ Reverse equation (b) CO2 (g) → CO(g) + 1 /2 O2 (g) ΔH = +283 kJ C(s) + 1 /2 O2 (g) → CO(g) ΔH = -111 kJ add this to (a) Notice that the enthalpy change represented by ΔH 3 (equation b) had to be reversed in solving for ΔH2 (the target equation). The value of the enthalpy change therefore changed from -283 kJ mol -1 to +283 kJ mol-1 . In the same way, if an equation is multiplied or divided, the value of ΔH should be treated in the same way. Q61: Since: C(s) + 1 /2 O2 (g) → CO(g) ΔH = -111 kJ mol-1 What would be the value (in kJ) for the enthalpy change for this reaction? Remember to include the sign. 1 / CO(g) → 1 / C(s) + 1 / O (g) ΔH = ? kJ 2 2 4 2 .......................................... Q62: Since: C(s) + 1 /2 O2 (g) → CO(g) ΔH = -111 kJ What would be the value (in kJ) for the enthalpy change for this reaction? Remember to include the sign. 3CO(g) → 3C(s) + 3 /2 O2 (g) ΔH = ? kJ .......................................... Key point Algebraic calculations from balanced equations can be used to calculate enthalpy changes for reactions which are difficult to measure. .......................................... In both of the methods employed in calculating enthalpy changes by application of Hess's law, the same rules have been used. © H ERIOT-WATT U NIVERSITY 116 TOPIC 3. CHEMICAL ENERGY • If an equation is reversed, then the sign of ΔH also changes. • If an equation is multiplied the value of ΔH should be multiplied by the same number. • If an equation is divided the value of ΔH should be divided by the same number. e.g. Knowing the value for (1), we can write equations like (2) and (3). equation (1) C(s) + O2 (g) → CO2 (g) ΔH = -394 kJ equation (2) 2C(s) + 2O2 (g) → 2CO2 (g) ΔH = -798 kJ equation (3) 1 / CO (g) 2 2 → 1 /2 C(s) + 1 /2 O2 (g) ΔH = +197 kJ Key point Enthalpy changes can be calculated by application of Hess's law. Calculations can be carried out using enthalpy cycle diagrams and/or by using algebraic calculations from balanced equations. 3.6 Bond enthalpies You saw earlier that you can think of the reaction between nitrogen and hydrogen to make ammonia as taking place by breaking all the bonds to produce atoms and then recombining these in a new way to make ammonia. The reaction does not occur that way; but now you know Hess's law, it is irrelevant how a reaction occurs; to calculate a reaction enthalpy change you only need to consider the starting reactants and the finishing products. So you can calculate the reaction enthalpy change in that way. The overall enthalpy change of reaction will be given by the total enthalpy of new bonds minus the enthalpy of the original bonds. Because they are so useful in thermodynamic calculations, chemists have produced tables of bond enthalpies. These can be found in your SQA data booklet. The enthalpy change values in these tables are always quoted for breaking one mole of bonds for gaseous reactants and products, and are always positive. (No bond will produce energy when it breaks.) For example, for a symmetrical bond such as H - H, the bond enthalpy is: H2 (g) → 2H(g) ΔH = +436 kJ mol-1 This bond enthalpy can only be for a hydrogen molecule, so the figure obtained should © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY 117 be exact. This will apply to other diatomic molecules where no other atoms are involved: for example, Cl2 (and other halogens), O=O, N≡N. These bond enthalpies are listed in one column of the SQA Data Booklet. For bonds between different atoms, for example, C - H: C - H(g) → C(g) + H(g) ΔH = +412 kJ mol-1 In this case the values obtained differ slightly depending on the environment of the bond. For example, the C - H bond enthalpy in methane (CH 4 ) will differ slightly from that in ethene (CH2 = CH2 ). The bond enthalpies are given as average bond enthalpies from several different reactants, in a separate table. You will sometimes see bond enthalpies (or energies) written as: E(X - Y) for the bond enthalpy for a X - Y bond. Bond enthalpies and the synthesis of ammonia We can now apply bond enthalpy data to the synthesis of ammonia: Go online N2 (g) + 3H2 (g) → 2NH3(g) Q63: Use the bond enthalpy data to construct a table showing enthalpy changes when bonds are broken and when bonds are formed. ΔH Bonds broken N≡N ? 3×H-H ? Total enthalpy change to break bonds ? Bonds formed ΔH 6×N-H ? Total enthalpy change on making bonds ? Then calculate the overall enthalpy change for this reaction as the sum of these two changes. .......................................... The enthalpy of formation (ΔH f ) of a compound is the enthalpy change of reaction when one mole of the compound is formed from its elements. In this reaction two moles of ammonia have been made from its elements (nitrogen and hydrogen). The enthalpy of formation of ammonia will be: (-75)/2 = -37.5 kJ mol-1 A quoted value for ΔH f of ammonia is -46.1 kJ mol-1 . Although the enthaply changes for breaking the H - H and N ≡ N bonds applied to the actual reactants (H 2 and N2 ), the N - H bond value quoted is a mean and this has led to the value calculated using bond enthalpies being lower than the actual value. .......................................... © H ERIOT-WATT U NIVERSITY 118 TOPIC 3. CHEMICAL ENERGY The hydrogenation of ethene The next series of questions concerns the hydrogenation of ethene to form ethane. Go online CH2 = CH2 + H2 → CH3 -CH3 The following bond enthalpies may be useful. Bond Bond enthalpy /kJ mol-1 H-H 436 (for H2 ) C-H 412 (average) C-C 348 (average) C=C 612 (average) Q64: Which type and number of bonds have to be broken? a) b) c) d) 1 x C=C only 1 x C=C and 1 x H - H 1 x C=C and 4 x C - H 1 x C=C, 4 x C - H and 1 x H - H .......................................... Q65: Which type and number of bonds have to be made? .......................................... Q66: Using the data provided, calculate (i) the enthalpy change to break the bonds, and (ii) the enthalpy change from forming bonds. Hence, calculate the enthalpy change for this reaction in kJ. .......................................... Q67: The enthalpy change found directly for this reaction is -136.9 kJ. Why are these results different? .......................................... 3.7 Summary Summary Chemical Energy • For industrial processes, it is essential that chemists can predict the quantity of heat energy taken in or given out. • Exothermic changes cause heat to be released to the surroundings. © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY Summary continued • Endothermic changes cause absorption of heat from the surroundings. • If reactions are endothermic, costs will be incurred in supplying heat energy in order to maintain the reaction rate. • If reactions are exothermic, the heat produced may need to be removed to prevent the temperature rising. Enthalpy • Chemical energy is also known as enthalpy. • The change in chemical energy associated with chemical reactions can be measured. • The enthalpy change is the energy difference between products and reactants. • The enthalpy change can be calculated from a potential energy diagram. • The enthalpy change has a negative value for exothermic reactions and a positive value for endothermic reactions. • The specific heat capacity, mass and temperature can be used to calculate the enthalpy change for a reaction. • The enthalpy of combustion of a substance is the enthalpy change when one mole of the substance burns completely in oxygen. • These values can often be directly measured using a calorimeter and values for common compounds are available from data books and online databases for use in Hess’s law calculations. • Enthalpy of solution is the energy change when one mole of a substance dissolves in water. The units are kJ mol-1 . • The enthalpy of neutralisation of an acid is defined as the energy change when it is neutralised to form 1 mole of water. The units are kJ mol -1 . Hess’s Law • Hess's law is used to calculate enthalpy change values that would be difficult to measure by experiment or cannot be measured directly because the reaction does not occur under normal conditions. • Hess’s law states that the enthalpy change for a chemical reaction is independent of the route taken, providing the starting point and finishing point is the same for both routes. • Enthalpy changes can be calculated by applying Hess’s law. • Enthalpy changes can be calculated by application of Hess's law. Calculations can be carried out using enthalpy cycle diagrams and/or by using algebraic calculations from balanced equations. © H ERIOT-WATT U NIVERSITY 119 120 TOPIC 3. CHEMICAL ENERGY Summary continued • Algebraic calculations from balanced equations can be used to calculate enthalpy changes for reactions which are difficult to measure. ◦ If an equation is reversed, then the sign of ΔH also changes. ◦ If an equation is multiplied the value of ΔH should be multiplied by the same number. ◦ If an equation is divided the value of ΔH should be divided by the same number. e.g. Knowing the value for (1), we can write equations like (2) and (3). equation (1) C(s) + O2 (g) → CO2 (g) ΔH = -394 kJ equation (2) 2C(s) + 2O2 (g) → 2CO2 (g) ΔH = -798 kJ equation (3) 1/2 C(s) + 1/2O 2 (g) → 1/2CO2(g) ΔH = -197 kJ Bond Enthalpies 3.8 • • For a diatomic molecule, XY, the molar bond enthalpy is the energy required to break one mole of XY bonds. • Mean molar bond enthalpies are average values which are quoted for bonds which occur in different molecular environments. • Bond enthalpies can be used to estimate the enthalpy change occurring for a gas phase reaction by calculating the energy required to break bonds in the reactants and the energy released when new bonds are formed in the products. Resources Higher Chemistry for CfE: J Anderson, E Allan and J Harris, Hodder Gibson, ISBN 978-1444167528 © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY 3.9 121 End of topic test End of topic 3 test This end of topic test is available online. If you do not have access to the internet, here is a paper version. In a reaction, the enthalpy of the reactants is +50 kJ mol -1 and the enthalpy of the products is -250 kJ mol-1 . Q68: Which equation should you use to calculate the enthalpy change for the reaction? A) ΔH = H(products) - H(reactants) B) ΔH = H(products) + H(reactants) C) ΔH = H(reactants) - H(products) D) ΔH = H(reactants) + H(products) .......................................... Q69: What is the value of ΔH for this reaction? A) -300 B) -200 C) +200 D) +300 .......................................... Q70: The reaction is: A) endothermic B) exothermic .......................................... Q71: Which equation illustrates an enthalpy of combustion? A) C2 H5 OH(l) + O2 (g) → CH3 COOH(l) + H2 O(l) B) C2 H6 (g) + 31 /2 O2 (g) → 2CO2 (g) + 3H2 O(l) C) CH4 (g) + 11 /2 O2 (g) → CO(g) + 2H2 O(l) D) CH3 CHO(l) + 1 /2 O2 (g) → CH3 COOH(l) .......................................... © H ERIOT-WATT U NIVERSITY Go online 122 TOPIC 3. CHEMICAL ENERGY Q72: When 3.6 g of butanal (relative formula mass = 72) was burned, 134 kJ of energy was released. From this result, what is the enthalpy of combustion of butanal? A) - 2680 kJ mol-1 B) - 6.7 kJ mol-1 C) + 6.7 kJ mol-1 D) + 2680 kJ mol-1 .......................................... Q73: Ethanol (C2 H5 OH) has a different enthalpy of combustion from dimethyl ether (CH3 OCH3 ). This is because the compounds have different: A) products of combustion. B) bonds within the molecules. C) molecular masses. D) boiling points. .......................................... Q74: The potential energy diagram for the reaction CO(g) + NO 2 (g) → CO2 (g) + NO(g) is shown. What is the ΔH for the reaction? a) - 361 kJ mol-1 © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY b) - 227 kJ mol-1 c) - 93 kJ mol-1 d) + 361 kJ mol-1 .......................................... The enthalpies of combustion of methanol, ethanol and propan-1-ol are given below. Methanol CH 3 OH ΔHc = -726 kJ mol-1 Ethanol C2 H5 OH ΔHc = -1367 kJ mol-1 Propan-1-ol C3 H7 OH ΔHc = -2021 kJ mol-1 Q75: Why is there a regular increase in enthalpies of combustion from methanol to ethanol to propan-1-ol? .......................................... Q76: What would you predict the enthalpy of combustion of butan-1-ol to be? .......................................... Q77: Identify the endothermic reactions from the thermochemical equations below. A) Ba(OH)2 (aq) + 2HCl(aq) → BaCl2 (aq) + 2H2 O(l) ; ΔH = -113.5 kJ mol-1 B) 3O2 (g) → 2O3 (g) ; ΔH = +142.7 kJ mol-1 C) NaOH(s) → Na+ (aq) + OH- (aq) ; ΔH = -42.7 kJ mol-1 D) C3 H8 (g) + 5O2 (g) → 3CO2 (g) + 4H2 O(g) ; ΔH = -2219 kJ mol-1 E) NH4 Cl (s) → NH4 + (aq) + Cl- (aq) ; ΔH = +15.0 kJ mol-1 F) 2Na(s) + Cl2 (g) → 2NaCl(s) ; ΔH = -411.1 kJ mol-1 .......................................... Q78: Identify the equations which apply to an enthalpy of solution. A) Ba(OH)2 (aq) + 2HCl(aq) → BaCl2 (aq) + 2H2 O(l) ; ΔH = -113.5 kJ mol-1 B) 3O2 (g) → 2O3 (g) ; ΔH = +142.7 kJ mol-1 C) NaOH(s) → Na+ (aq) + OH- (aq) ; ΔH = -42.7 kJ mol-1 D) C3 H8 (g) + 5O2 (g) → 3CO2 (g) + 4H2 O(g) ; ΔH = -2219 kJ mol-1 E) NH4 Cl (s) → NH4 + (aq) + Cl- (aq) ; ΔH = +15.0 kJ mol-1 F) 2Na(s) + Cl2 (g) → 2NaCl(s) ; ΔH = -411.1 kJ mol-1 .......................................... Q79: Identify the reaction which would be best able to provide heat to an environment. © H ERIOT-WATT U NIVERSITY 123 124 TOPIC 3. CHEMICAL ENERGY A) Ba(OH)2 (aq) + 2HCl(aq) → BaCl2 (aq) + 2H2 O(l) ; ΔH = -113.5 kJ mol-1 B) 3O2 (g) → 2O3 (g) ; ΔH = +142.7 kJ mol-1 C) NaOH(s) → Na+ (aq) + OH- (aq) ; ΔH = -42.7 kJ mol-1 D) C3 H8 (g) + 5O2 (g) → 3CO2 (g) + 4H2 O(g) ; ΔH = -2219 kJ mol-1 E) NH4 Cl (s) → NH4 + (aq) + Cl- (aq) ; ΔH = +15.0 kJ mol-1 F) 2Na(s) + Cl2 (g) → 2NaCl(s) ; ΔH = -411.1 kJ mol-1 .......................................... A pupil wanted to measure the enthalpy of combustion of butane. She used a camping stove to heat a pot of water and found that 10 g of butane burned to give out 258.6 kJ of energy. Q80: Write a balanced equation to show the reaction which corresponds to the enthalpy of combustion of butane. .......................................... Q81: Apart from the mass of the butane cylinder at the start and the end of the experiment, state three measurements which the pupil would have made. .......................................... Q82: Calculate the experimental value for the enthalpy of combustion of butane. Give your answer to the nearest kJ mol-1 , and remember the sign. .......................................... Q83: Enthalpy changes for a reaction which can be carried out by different routes must be: A) exothermic. B) opposite. C) the same. D) endothermic. .......................................... The enthalpy of combustion of carbon monoxide is -283 kJ mol -1 . Q84: What is the enthalpy change if 2.8 g of carbon monoxide is burned? .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY Q85: What is the enthalpy change when 88 g of carbon dioxide is converted to CO and oxygen? .......................................... Q86: Look at the enthalpy cycle diagram below. What is the relationship between the enthalpy changes? a) b) c) d) ΔH3 ΔH3 ΔH2 ΔH1 = ΔH1 = ΔH2 = ΔH3 = ΔH2 - ΔH2 + ΔH1 - ΔH1 - ΔH3 .......................................... Q87: What is the enthalpy change for ΔH 3 ? .......................................... The questions below refer to the following reaction. Si2 H6 (g) → 2Si(s) + 3H2 (g), ΔH = -78 kJ Q88: What is the enthalpy change for the reaction below? 2Si2 H6 (g) → 4Si(s) + 6H2 (g) .......................................... Q89: What is the enthalpy change for this reaction? Si(s) + 3 /2 H2 (g) → 1 /2 Si2 H6 (g) .......................................... Using the information below: a) 2Fe(s) + 3 /2 O2 (g) → Fe2 O3 (s), ΔH = - 827 kJ b) 2Al(s) + 3 /2 O2 (g) → Al2 O3 (s), ΔH = - 1675 kJ © H ERIOT-WATT U NIVERSITY 125 126 TOPIC 3. CHEMICAL ENERGY Q90: Calculate the enthalpy change for the reaction: 2Al(s) + Fe2 O3 (s) → Al2 O3 (s) + 2Fe(s) .......................................... Q91: Given the enthalpy changes for the three reactions below: a) O2 (g) → 2O(g), ΔH = +496 kJ b) C(s) → C(g), ΔH = + 715 kJ c) C(s) + O2 (g) → CO2 (g), ΔH = - 394 kJ Calculate the enthalpy change for the reaction: C(g) + 2O(g) → CO2 (g) .......................................... In this diagram ΔH is the enthalpy change involved in the formation of one mole of ethanol from carbon, hydrogen and oxygen. Other enthalpy changes involved in the cycle are the enthalpies of combustion of carbon, hydrogen and ethanol, which are available from the data booklet. Q92: What is the enthalpy value, X, for the reaction 2C(s) + aO 2 (g) → 2CO2 (g)? .......................................... Q93: What is the enthalpy value, Y, for the reaction 3H 2 (g) + bO2(g) → 3H2 O(l)? .......................................... Q94: What is the value of c in the reaction 2CO 2 (g) + 3H2 O(l) - cO2 (g) → C2 H5 OH(l)? .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 3. CHEMICAL ENERGY 127 Q95: What is the enthalpy value, Z, for the reaction 2CO 2 (g) + 3H2 O(l) - cO2 (g) → C2 H5 OH(l)? .......................................... Q96: Hence, what is the enthalpy value, ΔH, for the reaction 2C(s) + 3H 2 (g) + 1/2 O2 (g) → C2 H5 OH(l)? .......................................... Q97: The mean bond enthalpy of the N-H bond is equal to one third of the value of ΔH for which change? a) b) c) d) N(g) + 3H(g) → NH3 (g) N2 (g) + 3H2 (g) → 2NH3(g) 0.5N2 (g) + 1.5H2 (g) → NH3 (g) NH3 (g) → 0.5N2 (g) + 1.5H2 (g) .......................................... Q98: The table below contains information about some diatomic molecules. Bond H-H ◦ Boiling point C -253 Bond point (kJ 436 mol-1 ) H-CI -85 CI-CI -35 Br-Br 59 432 243 194 Which of the diatomic molecules listed has the strongest covalent bond? a) b) c) d) H-H H-CI CI-CI Br-Br .......................................... Q99: Bond Enthalpy (kJ mol-1 ) CI-CI C-CI C-H H-CI 243 338 412 432 Chloromethane can be produced by the reaction of methane with chlorine. CH4 (g) + Cl2 (g) → CH3 Cl(g) + HCl(g) Using the information in the table above, calculate the enthalpy change, in kJ mol-1 , for this reaction. a) b) c) d) -3897 -115 +115 +3897 © H ERIOT-WATT U NIVERSITY 128 TOPIC 3. CHEMICAL ENERGY .......................................... Q100: Use bond enthalpy values from the data book to calculate the enthalpy change for the following reaction. (Hint: remember the sign.) CH4 (g) + Br2 (g) → CH3 Br (g) + HBr (g) .......................................... Q101: The data book gives the enthalpy of combustion of methane as -891 kJ mol -1 Use bond enthalpies to calculate the enthalpy change for this reaction. (Hint: remember the sign.) CH4 (g) + 2O2 (g) → CO2 (g) + 2H2 O (g) .......................................... Q102: Using bond enthalpy values, calculate the enthalpy change for the following addition reaction. C2 H4 (g) + HBr (g) → C2 H5 Br (g) .......................................... .......................................... © H ERIOT-WATT U NIVERSITY 129 Topic 4 Oxidising or reducing agents Contents 4.1 Prior knowledge . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 132 4.2 4.3 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Elements as oxidising and reducing agents . . . . . . . . . . . . . . . . . . . . 133 134 4.4 4.3.1 Displacement reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . Molecules and group ions as oxidising and reducing agents . . . . . . . . . . . 138 139 4.5 4.4.1 Oxygen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Uses for strong oxidising agents . . . . . . . . . . . . . . . . . . . . . . . . . . 140 142 4.6 4.7 Ion-electron half equations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Combining ion-electron equations . . . . . . . . . . . . . . . . . . . . . . . . . 145 148 4.8 4.7.1 Tutorial - simple ion-electron equations . . . . . . . . . . . . . . . . . . Complex ion-electron equations . . . . . . . . . . . . . . . . . . . . . . . . . . 149 150 4.9 4.8.1 Tutorial - complex ion-electron equations . . . . . . . . . . . . . . . . . Redox titrations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 153 155 4.10 Summary cloze test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.11 Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 159 161 4.12 Resources . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 162 4.13 End of topic test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 163 Prerequisite knowledge You should already know that: • balanced ionic equations can be written to show the reaction of metals with water, oxygen and acids (National 5, Unit 3); • ion-electron equations can be written for electrochemical cells including those involving non-metals (National 5, Unit 3); • combinations of these reactions form redox equations (National 5, Unit 3); • spectator ions can be described and identified from equations (National 5, Unit 3); • students should be able to explain the principles of and carry out an acid / base titration (National 5, Unit 3). 130 TOPIC 4. OXIDISING OR REDUCING AGENTS Learning objectives By the end of this topic you should be able to state that: Elements as oxidising or reducing agents • A redox reaction is a reaction in which reduction and oxidation occur together, reduction being the gain of electrons by a reactant and oxidation being the loss of electrons by a reactant in a reaction. • An oxidising agent is a substance which accepts electrons. • A reducing agent is a substance which donates electrons. • Oxidising and reducing agents can be identified in redox reactions. • Elements with low electronegativities (metals) tend to form ions by losing electrons (oxidation) and so can act as reducing agents. • Elements with high electronegativities (non-metals) tend to form ions by gaining electrons (reduction) and so can act as oxidising agents. • The strongest reducing agents are found in Group 1. • The strongest oxidising agents come from Group. • The electrochemical series indicates the effectiveness of oxidising and reducing agents. Compounds as oxidising or reducing agents • Compounds can also act as oxidising or reducing agents. • Electrochemical series contain a number of ions and molecules. • The dichromate and permanganate ions are strong oxidising agents in acidic solutions whilst hydrogen peroxide is an example of a molecule which is a strong oxidising agent. • Carbon monoxide is an example of a gas that can be used as a reducing agent. • Oxidising and reducing agents can be selected using an electrochemical series from a data booklet or can be identified in the equation showing a redox reaction. Use of oxidising agents • Oxidising agents are widely employed because of the effectiveness with which they can kill fungi and bacteria, and can inactivate viruses. • The oxidation process is also an effective means of breaking down coloured compounds making oxidising agents ideal for use as ‘bleach’ for clothes and hair. Ion-electron equations © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS • Oxidation and reduction reactions can be represented by ion-electron equations. • When molecules or group ions are involved, if the reactant and product species are known, a balanced ion-electron equation can be written by adding appropriate numbers of water molecules, hydrogen ions and electrons. • Ion-electron equations can be combined to produce redox equations. Practical applications • Displacement reactions are example of redox reactions and oxidising and reducing agents can be identified in these and other redox reactions. • The technique of titration can be applied to redox reactions, allowing the concentration of a reactant to be calculated from results of volumetric titrations. © H ERIOT-WATT U NIVERSITY 131 132 TOPIC 4. OXIDISING OR REDUCING AGENTS 4.1 Prior knowledge Test your prior knowledge Q1: Go online a) b) c) d) Reduction can be defined as: gain of electrons. loss of electrons. loss in mass of a substance. gain in mass of a substance. .......................................... Q2: Potassium iodide will react with lead nitrate to produce a precipitate of lead iodide. Which of the following best represents this? a) b) c) d) 2Pb+(aq) + 2NO3 - (aq) + 2K+ (aq) + 2l- (aq) → 2Pb+(l- )2 (s) + 2K+ (aq) + 2NO3 - (aq) Pb+ (aq) + 2NO3 - (aq) + 2K+ (aq) + 2l- (aq) → Pb2+ (l- )2 (aq) + 2K+ (aq) + 2NO3 - (aq) Pb2+ (aq) + 2NO3 - (aq) + 2K+ (aq) + 2l- (aq) → Pb2+ (l- )2 (s) + 2K+ (aq) + 2NO3 - (aq) Pb2+ (NO3 - )2 (aq) + 2K+ l- (aq) → Pb2+ (l- )2 (s) + 2K+ NO3 - (aq) .......................................... Q3: Combine the following reactions to give the overall redox reaction. Oxidation H2 O2 (l) → O2 (g) + 2H+ (aq) + 2eReduction 5e- + MnO4 - (aq) + 8H+ (aq) → Mn2+ (aq) + 4H2 O(l) a) H2 O2 (l) + 5e- + MnO4 - (aq) + 8H+ (aq) → Mn2+ (aq) + 4H2 O(l) + O2 (g) + 2H+ (aq) + 2eb) H2 O2 (l) + MnO4 - (aq) + 8H+ (aq) → Mn2+ (aq) + 4H2 O(l) + O2 (g) + 2H+ (aq) c) 5H2 O2 (l) + 10e- + 2MnO4- (aq) + 16H+ (aq) → 2Mn2+(aq) + 8H2 O(l) + 5O2 (g) + 10H+(aq) + 10ed) 5H2 O2 (l) + 2MnO4 - (aq) + 16H+ (aq) → 2Mn2+ (aq) + 8H2 O(l) + 5O2 (g) + 10H+(aq) e) 5H2 O2 (l) + 2MnO4 - (aq) + 6H+ (aq) → 2Mn2+(aq) + 8H2 O(l) + 5O2 (g) .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS 4.2 Introduction The term 'redox' is an abbreviation of the chemical reactions commonly known as reduction and oxidation. These processes occur together and include many common reactions such as the burning of fossil fuels, the corrosion of metals and photosynthesis. Rusting is a common redox reaction in which iron is converted into iron oxides. Photosynthesis is another common redox reaction, involving both reduction and oxidation stages. An early definition of reduction describes the removal of oxygen from metal oxides. The metal oxide is said to be reduced to the metal. An example is iron(III) oxide being reduced to iron. Iron oxide reduction An early definition of oxidation describes the addition of oxygen to a metal forming a metal oxide. A familiar example is the rusting of iron to give iron(III) oxide. In this case the iron metal is being oxidised. Iron oxidation Iron(III) oxide consists of Fe3+ and O2- ions. Close analysis of the above reaction shows that the iron atoms have had electrons removed from them, and these electrons are transferred to the oxygen. The iron has been oxidised and the oxygen has been reduced. The changes can be shown clearly by writing ion-electron half equations for each reaction. © H ERIOT-WATT U NIVERSITY 133 134 TOPIC 4. OXIDISING OR REDUCING AGENTS Iron oxide half equations Notice that the equations have been balanced by ensuring that the number of atoms on each side of the arrow is the same and that the number of electrons exchanged between the two half equations is the same. A useful memory aid for the definitions of oxidation and reduction is provided by the first letters in the phrase "OIL RIG" (see below). Oxidation is Loss of electrons. Reduction is Gain of electrons. 4.3 Elements as oxidising and reducing agents The corrosion of iron results in the formation of iron(III) oxide (see below). Iron oxidation 2 As already seen, this reaction can be represented by two ion-electron equations (see below). © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS Iron oxide half equations 2 The iron metal is being oxidised by the oxygen. Another way of expressing this is to say that the oxygen is acting as an oxidising agent. The oxygen is receiving electrons from the iron. An oxidising agent is therefore an electron acceptor. In a similar way, the oxygen molecules are being reduced by the iron. The iron is acting as a reducing agent. The iron is donating electrons to the oxygen. A reducing agent is therefore an electron donor. Sometimes there are ions present which do not react in the redox reaction. These ions are present in the reactants and are still present in the same amount when the products have formed. Such ions are known as spectator ions and are not normally shown in the balanced redox equation. The Electrochemical Series (also known as the Table of Standard Reduction Potentials) can be found in your SQA Data Book and can give an indication of the strength of oxidising and reducing agents. The easiest reductions occur at the foot of the series, left to right. The best oxidising agents are therefore found at the bottom left side. Similarly the easiest oxidations occur at the top of the series, right to left . This is summarised below. Reducing and oxidising agents © H ERIOT-WATT U NIVERSITY 135 136 TOPIC 4. OXIDISING OR REDUCING AGENTS Example exercise When bromine is added to potassium iodide solution, iodine is displaced and potassium bromide solution is formed (see below). Potassium bromide formation The two potassium ions are unchanged in this reaction. These are spectator ions, and ignoring these ions results in the ion electron equations as shown below. Iodide and bromine half equations Q4: Is the first ion-electron equation (see above - Iodide and bromine half equations) an oxidation or a reduction? .......................................... Q5: a) b) c) d) Which of these is acting as an oxidising agent? Iodide Iodine Bromine Bromide .......................................... Q6: Name the chemical species acting as a reducing agent. .......................................... Q7: Where in the Electrochemical Series in the data booklet will you find the reaction which is most likely to go in the opposite direction? a) Top of the table. b) Bottom of the table. .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS Q8: Use this equation showing a redox change in this and the next two questions. Name the reactant being reduced. .......................................... Q9: Name the reactant acting as an electron donor. .......................................... Q10: Name the oxidising agent. .......................................... Q11: Which of the following is the best oxidising agent? a) b) c) d) e) f) Na (s) Br2 (1) Sn2+ (aq) I- (aq) Zn (s) Cu2+ (aq) .......................................... Q12: Which of the following is the best reducing agent? a) b) c) d) e) f) Na (s) Br2 (1) Sn2+ (aq) I- (aq) Zn (s) Cu2+ (aq) .......................................... Q13: Which substance could be used as an oxidising agent or a reducing agent? a) b) c) d) e) f) Na (s) Br2 (1) Sn2+ (aq) I- (aq) Zn (s) Cu2+ (aq) .......................................... © H ERIOT-WATT U NIVERSITY 137 138 TOPIC 4. OXIDISING OR REDUCING AGENTS 4.3.1 Displacement reactions Displacement reactions Go online A strip of zinc metal is placed into copper(II) sulfate solution. The zinc ionises and passes into solution, and copper ions plate onto the surface of the strip as copper atoms, replacing the zinc. The solution loses its blue colour since the resulting zinc sulfate solution is colourless. Zinc metal Copper Sulfate solution 2+ 2+ Cu SO4 2+ SO4 Zn 2+ 2+ SO Zn Zn 2+ Cu Zn 2+ 2+ SO4 Zn Cu 2+ Cu 2+ SO4 2+ Zn 2+ Cu Zn Zn Cu 2+ Cu 2+ 4 SO 2+ Cu SO4 Zn2+ SO4 2+ Zn Cu SO4 2+ Zn Cu 2+ 4 2+ Cu 2+ 4 SO Cu 2+ 4 SO 2+ SO4 Zn2+ Zinc metal Copper metal Zinc Sulfate solution Q14: Name the spectator ion in this reaction. .......................................... Q15: Complete the ion-electron half equation for the oxidation taking place: Zn → .......................................... Q16: Complete the ion-electron half equation for the reduction taking place: → Cu .......................................... Q17: Which of these is acting as an oxidising agent? © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS a) b) c) d) Zinc atoms Zinc ions Copper atoms Copper ions .......................................... Q18: Name the reactant acting as an electron donor. .......................................... Key point An oxidising agent is a substance which accepts electrons; a reducing agent is a substance which donates electrons. Oxidising and reducing agents can be identified in redox reactions. .......................................... 4.4 Molecules and group ions as oxidising and reducing agents You might have noticed that as well as elements being listed in the table of Standard Reduction Potentials, there are some compounds. Dichromate ion (Cr 2 O7 2- ) and permanganate ion (MnO 4 - ) are commonly used as oxidising agents in chemical reactions. You will study the reactions of permanganate and dichromate ions in more detail in a later section. Q19: Can you think of a practical reason why sodium dichromate or potassium permanganate are frequently used as oxidising agents in chemistry as opposed to the elements studied so far? .......................................... Q20: For example, a traditional method for producing chlorine is the reaction between concentrated hydrochloric acid and potassium permanganate. The formulae for the reactants and products are: KMnO4 + HCl → KCl + MnCl2 + H2 O + Cl2 It is quite difficult, but can you balance this equation? .......................................... Q21: Remembering that KMnO 4 consists of K+ and MnO4 - ions and HCl is also ionised in solution, what is the oxidising agent in this reaction, and what are the reducing agents? .......................................... © H ERIOT-WATT U NIVERSITY 139 140 TOPIC 4. OXIDISING OR REDUCING AGENTS Q22: Look carefully at the reactants and products again. What are the products from these oxidising and reducing agents? .......................................... Q23: Finally, are there any spectator ions in this reaction? .......................................... 4.4.1 Oxygen Almost all life on Earth depends on oxygen to provide energy. When the Earth was formed 4.5 billion (4.5 x 10 9 ) years ago* it was a very different place. As the hot fireball cooled it formed a solid crust, volcanoes belched out gases: steam, carbon dioxide and ammonia. These formed the oceans and an atmosphere with no oxygen. We now have an atmosphere consisting of about 21% oxygen. The rest is mainly nitrogen. Here is our beautiful blue planet. © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS The change was brought about somewhere around 2.7 to 2.2 billion years ago by vast numbers of cyanobacteria (blue-green algae) in the oceans. These organisms used the energy of sunlight in photosynthesis to convert the abundant supplies of carbon dioxide and water into carbohydrates and oxygen. This dramatic change in atmospheric composition fuelled the abundant numbers of plant and animal species which now use oxygen to provide energy for life. Q24: A simple equation for photosynthesis, making glucose, C 6 H12 O6 , is: 6CO2 + 6H2 O + light energy → C6 H12 O6 + 6O2 Can you see in this case what is oxidised and what is reduced? .......................................... Q25: When we humans use glucose to provide energy for life, the equation is simply reversed: C6 H12 O6 + 6O2 → 6CO2 + 6H2 O + energy The atmospheric concentration of carbon dioxide measured at Mauna Loa, Hawaii, has risen from 315 ppmv in 1960 to 390 ppmv in 2010. ppmv is parts per million by volume; μ CO2 per atmosphere. Assuming the 'effective volume' of Earth's atmosphere is 4 x 1020 , what volume of additional carbon dioxide has the atmosphere contained in going from 1960 to 2010? .......................................... [* Evidence for the age of the Earth comes mainly from studying the ages of rocks using a variety of radioactive decay data.] © H ERIOT-WATT U NIVERSITY 141 142 TOPIC 4. OXIDISING OR REDUCING AGENTS 4.5 Uses for strong oxidising agents The main domestic and industrial uses of strong oxidising agents are as biocides and bleaches. Sodium chlorate(l) solution This weedkiller contains is a powerful germicide and sodium chlorate(lll) bleach. The original washing detergents contained perborates, percarbonates and silicates, from which Persil derived its name. Biocides Biocides are used for killing fungi and bacteria, and inactivating viruses. The main oxidising agents used are: chlorine, hypochlorite, chlorine dioxide, iodine, and permanganate. Disinfection during a foot-and-mouth outbreak using hypochlorite Disinfection with iodine during a bone marrow donation © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS 143 Bleaches These are used for bleaching textiles, paper and hair. Common oxidising agents include: sulfite, chlorine and peroxides. Perborate is used for adding extra whiteness to laundry and dibenzoyl peroxide for treating spots and acne. The original washing detergents contained perborates, percarbonates and silicates, from which Persil derived its name. Gel for acne Water purification Water from reservoirs is purified considerably before it is pumped into buildings for consumption. As well as removing particulate matter there are possible pathogens to be removed. These comprise viruses, bacteria, including Escherichia coli, Campylobacter and Shigella, and protozoa, including Giardia lamblia and other cryptosporidia. The most common disinfection method involves some form of chlorine or its compounds such as chloramine or chlorine dioxide. Chlorine dioxide is a faster-acting disinfectant than elemental chlorine, however it is relatively rarely used, because in some © H ERIOT-WATT U NIVERSITY 144 TOPIC 4. OXIDISING OR REDUCING AGENTS circumstances it may create excessive amounts of chlorite.The use of chloramine is becoming more common as a disinfectant. Although chloramine is not as strong an oxidant, it does provide a longer-lasting residual than free chlorine and it won't form undesirable by-products. Ozone (O 3 ) is an unstable molecule which readily gives up one atom of oxygen providing a powerful oxidizing agent which is toxic to most waterborne organisms. It is a very strong, broad spectrum disinfectant that is widely used in Europe. For disinfection of small quantities of water when camping, for example, tablets which produce hypochlorite (chlorate(I)) in situ can be used. Propellants A further use for strong oxidants is as propellants for space rockets. These include hydrogen peroxide and liquid oxygen. For example, the Apollo-Saturn V first stage uses kerosene-liquid oxygen, the upper stages use liquid hydrogen-liquid oxygen. © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS 4.6 Ion-electron half equations When sodium burns in chlorine the product, sodium chloride, is an ionic compound. The reaction is a redox reaction and can be described by writing two ion-electron equations. The first shows the sodium (see below). Sodium ionising This ion-electron equation can have its charge balanced by showing the two electrons lost from the sodium atom on the right hand side of the equation (see below). Sodium ionising 2 Similarly, the chlorine ion-electron half equation can be balanced by showing the gain of two electrons by the chlorine on the left of the equation (see below). Chlorine half equations Practise writing ion-electron equations in this next activity. © H ERIOT-WATT U NIVERSITY 145 146 TOPIC 4. OXIDISING OR REDUCING AGENTS Writing ion-electron equations Go online Complete these oxidation and reduction half equations, then compare your answers with those at the end. Q26: Complete the ion electron half equation for the oxidation of potassium, forming a K+ ion: K→ .......................................... Q27: The oxidation of magnesium: Mg → .......................................... Q28: The oxidation of aluminium: Al → .......................................... Q29: Complete the ion-electron equation for the reduction of a fluorine atom to a fluoride ion: →F.......................................... Q30: The reduction of a nitrogen atom to nitride ion: → N3.......................................... Q31: The reduction of an oxygen atom to oxide ion: → O2.......................................... Many ion-electron equations do not have to be worked out as they can be found in a table known as the "Electrochemical Series: Standard Reduction Potentials". This is available in most chemistry texts or in data booklets. The oxidation ion-electron equation which applies can be obtained by reversing the reduction equation given in the table. Simple rules In a redox reaction a simple rule applies when determining which equation will be the reduction and which the oxidation. © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS The ion-electron equation appearing lowest in the electrochemical series will go as written (i.e. as a reduction), and this reaction will be able to force any ion-electron equation higher up in the table to go in reverse (i.e. as an oxidation). This can be described as the 'Anticlockwise Rule' (see below). Anticlockwise rule diagram Use the diagram (above) and the Electrochemical Series in your SQA Data Booklet to answer the following questions. Q32: In the Electrochemical Series shown, where will the easiest reduction be found? a) At the top of the table. b) At the bottom of the table. .......................................... Q33: In the Electrochemical Series shown, where will the easiest oxidation be found? a) b) c) d) At the top of the table, going "as written". At the bottom of the table, going "as written". At the top of the table, in reverse. At the bottom of the table, in reverse. .......................................... Q34: Which of the following reductions is the easiest? a) b) c) d) Na+ (aq) + e- → Na(s) F2 (g) + 2e- → 2F- (aq) Br2 (g) + 2e- → 2Br- (aq) Cu2+ (aq) + 2e- → Cu(s) .......................................... © H ERIOT-WATT U NIVERSITY 147 148 TOPIC 4. OXIDISING OR REDUCING AGENTS Q35: Which of these reactions would be reversed by the following reduction? 2H+ (aq) + 2e- → H2 (g) a) b) c) d) Na+ (aq) + e- → Na(s) F2 (g) + 2e- → 2F- (aq) Br2 (g) + 2e- → 2Br- (aq) Cu2+ (aq) + 2e- → Cu(s) .......................................... Key point Ion-electron equations can be written for oxidation and reduction reactions. Many ion-electron equations can be obtained from the Electrochemical Series available in a suitable Data Booklet. 4.7 Combining ion-electron equations The balanced redox equation for a chemical change can be worked out from the ion-electron equations which make up the oxidation and reduction stages. Often these can be found in the table of standard reduction potentials: the electrochemical series. This is available in most chemistry texts or in data booklets. The oxidation ion-electron equation which applies can be obtained by reversing the reduction equation given in the table. Writing the balanced redox equation can best be illustrated by use of an example. Example Problem: Sodium reacting with chlorine When sodium is placed into chlorine gas, the product, sodium chloride, is an ionic compound. Write a balanced redox equation from the two ion-electron equations. Solution Consider the two reactants (sodium and chlorine). The ion-electron equation appearing lowest in the electrochemical series will go as written (i.e. as a reduction). This is described in the 'Anticlockwise Rule' (see above). Chlorine to chloride In this reaction (above) the chlorine is the oxidising agent and will be able to force any of the ion-electron equations higher up in the table to go in reverse (i.e. as an oxidation). In this case sodium will be oxidised (see below). © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS 149 Sodium oxidation Combining the individual ion-electron equations into the redox equations requires a number of "balancing" stages. .......................................... Balancing redox equations The diagram below shows the stages required. Go online Balancing redox equations .......................................... 4.7.1 Tutorial - simple ion-electron equations Tutorial - simple ion-electron equations Use the electrochemical series to try these examples. In each case: Go online • write the ion-electron equation for the reduction. • write the ion-electron equation for the oxidation. • combine these to form the redox equation. © H ERIOT-WATT U NIVERSITY 150 TOPIC 4. OXIDISING OR REDUCING AGENTS Q36: When magnesium is placed into chlorine gas, the product, magnesium chloride, is an ionic compound. .......................................... Q37: When chlorine reacts with a solution containing iodide ions, iodine is one of the products. .......................................... Q38: Magnesium reacts with sulfuric acid to give hydrogen gas and magnesium sulfate solution. The spectator ions can be ignored when writing the redox equation. .......................................... Q39: Aluminium displaces silver ions from a solution of silver(I) nitrate, giving aluminium nitrate solution and silver. The spectator ions can be ignored when writing the redox equation. .......................................... Key point Ion-electron equations can be combined to produce redox equations. 4.8 Complex ion-electron equations Many of the more complex ion-electron equations involve hydrogen ions and/or water molecules in the chemical change. This is why reagents like "acidified" potassium permanganate or "acidified" potassium dichromate (see below) require an acid solution in which to act in redox reactions. Complex ion-electron equations Not all of these more complex ion-electron equations are listed in data booklets but, given the reactant and product species, ion-electron equations involving H + (aq) and H2 O(l) can be written. Example Acidified dichromate ions, Cr2 O7 2- , can be used to oxidise Fe 2+ ions. The Fe2+ ions are changed to Fe 3+ ions and the dichromate to chromium(III), Cr 3+ . © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS 151 Write ion-electron equations for each reaction and combine these to give a redox equation for the change. Complex equations Work out the balanced ion-electron equation for this next example using the steps (below) and then answer the questions. Complex equations The oxidation step involves the Fe 2+ ions being changed into Fe 3+ ions. The other steps in the diagram are:• STEP 1 The reduction given in the question can be written and the number of atoms in the given ions balanced. • STEP 2 Balance the number of oxygens by adding the correct number of water molecules. • STEP 3 Balance the number of hydrogen atoms by adding the correct number of hydrogen ions. • STEP 4 Balance the charge on each side of the equation by adding electrons to the most positive side. Writing the full redox equation requires the oxidation and reduction ion-electron equations to be combined . © H ERIOT-WATT U NIVERSITY Go online 152 TOPIC 4. OXIDISING OR REDUCING AGENTS Balance the number of electrons being transferred between the ion-electron equations and add them to give the redox equation. .......................................... Example Fe2+ ions can be changed into Fe 3+ ions in reaction with hypochlorite ions. The hypochlorite ion is converted to chloride (Figure 4.1). Figure 4.1: hypochlorite reduction .......................................... Write a balanced ion-electron equation for this change (Figure 4.1) using the steps shown above then answer the questions. Q40: How many water molecules have to be added to the product side? .......................................... Q41: How many hydrogen ions have to be added to the reactant side? .......................................... Q42: How many electrons have to be added to balance the charge? .......................................... Q43: On which side do the electrons get added? a) Reactant b) Product .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS 153 Q44: Is the completed ion-electron equation an oxidation or a reduction? a) Oxidation b) Reduction .......................................... Q45: Write a complete balanced redox equation for the reaction between hypochlorite and Fe2+ . .......................................... 4.8.1 Tutorial - complex ion-electron equations Tutorial - complex ion-electron equations In the first example, write a balanced ion-electron equation for the given change and then answer the questions which take the process step by step. Example 1 V3+ ions can be changed into VO 3- ions as part of a redox reaction. Write a balanced ion-electron equation for the change and then answer the questions which take the process step by step. Q46: How many water molecules have to be added to the reactant side? .......................................... Q47: How many hydrogen ions have to be added to the product side? .......................................... Q48: How many electrons have to be added to balance the charge? .......................................... Q49: On which side do the electrons get added? a) Reactant b) Product .......................................... Q50: Is the completed ion-electron equation an oxidation or a reduction? a) Oxidation b) Reduction .......................................... © H ERIOT-WATT U NIVERSITY Go online 154 TOPIC 4. OXIDISING OR REDUCING AGENTS In the second example, write a balanced ion-electron equation for the given change and then check your answer at the end. Example 2 Q51: ClO3 - ions can be changed into Cl 2 molecules as part of a redox reaction. Write a balanced ion-electron equation for the change. (Hint: balance the number of chlorine atoms first). .......................................... Use the electrochemical series in the data booklet to help with these examples. In each case: • write the ion-electron equation for the reduction. • write the ion-electron equation for the oxidation. • combine these to form the redox equation. Q52: When zinc is placed into sulfuric acid, the products are hydrogen gas and zinc sulfate solution. .......................................... Q53: In acid solution, potassium permanganate oxidises iron(II) to iron(III). .......................................... Q54: In dilute nitric acid solution, copper metal is oxidised to copper(II) ions. The nitrate ion is reduced to nitrogen monoxide gas. .......................................... .......................................... Key point Given reactant and product species, ion-electron equations which include H + (aq) and H2 O(l) can be combined to produce redox equations. © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS 4.9 Redox titrations Any balanced chemical equation can be used to calculate quantities of chemicals involved in the reaction. Knowing the quantity of one reactant can allow calculation of the quantity of another, as long as the end-point of the reaction can be observed. Titration of a solution of an oxidising agent against a reducing agent is known as a redox titration and, if a suitable indicator is available, can be used to determine the concentration of unknown reagents. For example: When acidified dichromate ions, Cr 2 O7 2- , are used to oxidise Fe2+ ions. The Fe2+ ions are changed to Fe 3+ ions and the dichromate to chromium(III), Cr 3+ . The redox equation is given below. This equation was fully developed in the example given in the previous page. Iron(III) dichromate redox The balanced redox equation (above) shows that 1 mole of dichromate ions (Cr 2 O7 2- ) is able to oxidise 6 moles of iron(II) ions (Fe2+ ). Example : Redox titration example Individual 20 cm3 portions of acidified Fe2+ solution of unknown concentration are titrated with 0.02 mol l -1 potassium dichromate solution, using a suitable indicator. Ignoring the first rough titration, the average volume of dichromate solution used was found to be 14.5 cm 3 . The results of the experiment and the balanced redox equation can be used to work out the concentration of the unknown Fe 2+ solution. © H ERIOT-WATT U NIVERSITY 155 156 TOPIC 4. OXIDISING OR REDUCING AGENTS .......................................... This type of calculation will be used in the next activity and in the tutorial examples. Estimation of vitamin C in fruit juices Go online Vitamin C (formula C6 H8 O6 ) is a vital dietary component found in fruit and vegetables. A lack of this vitamin in the diet leads to scurvy, a potentially fatal disease. The concentration of vitamin C in a sample can be determined in a redox titration using an iodine solution of known concentration and starch solution as indicator (see below). Vitamin C titration equations The on-line version of this activity contains a simulation experiment titrating three different fruit juices against a known concentration of iodine. If you do not have access to the on-line material, study this diagram (above) and text before trying the questions. © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS 157 A 20 cm3 portion of orange juice is pipetted into a flask and a few drops of starch solution added. Iodine solution of known concentration is added from the burette until, at the end point, the mixture turns a permanent blue/black colour. The first titration value (rough) is discarded and two or more further titrations are done until two volumes within 0.2 cm3 of each other are obtained. An average of these concordant titrations is noted and the process repeated for the other two types of fruit juice. The table of results is shown. GRAPE FRUIT Vitamin C titration Q55: Which fruit juice required the largest volume of iodine solution and has the highest concentration of vitamin C? .......................................... Q56: What volume of iodine solution (to 1 decimal place) was required to oxidise the vitamin C in each titration of lemon juice? .......................................... Q57: Given that the concentration of iodine solution used was 0.005 mol l -1 , calculate the number of moles of iodine used in each titration of the lemon juice. © H ERIOT-WATT U NIVERSITY 158 TOPIC 4. OXIDISING OR REDUCING AGENTS .......................................... Q58: Using the balanced redox equation for the reaction (see 'Vitamin C titration equations' above), which of these ratios shows the ratio of vitamin C to iodine? a) b) c) d) 1:1 1:2 2:1 6:2 .......................................... Q59: Given that the volume of iodine solution used in each titration was 20.0 cm 3 , use your answer to the last question to calculate the concentration of vitamin C in the lemon juice in mol l -1 . .......................................... Q60: One mole of vitamin C has a molar mass of 176 g mol -1 . concentration of the lemon juice solution in mg l -1 . Calculate the .......................................... Q61: The recommended daily allowance (RDA) of vitamin C suggested as being necessary to maintain good health, is 60 mg. What volume of lemon juice would be required to meet this requirement? .......................................... Tutorial - calculations in redox titrations Go online Q62: Return to the titration diagram and calculate the concentration of vitamin C in the orange juice in mol l -1 .......................................... Q63: One mole of vitamin C has a molar mass of 176 g mol -1 . concentration of the orange juice solution in mg l -1 . Calculate the .......................................... Q64: The recommended daily allowance (RDA) of vitamin C suggested as being necessary to maintain good health, is 60 mg. What volume of orange juice would be required to meet this requirement? .......................................... A worked solution to the next three questions can be accessed at the back of the book. Try to work out your answers on paper before accessing the answer at the end. Q65: The water in swimming pools can be kept sterile by adding chlorine, which kills micro-organisms. Chlorine levels in swimming pools can be monitored by titration with iron(II) sulfate. The related redox equation is shown below. © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS 159 Iron(II) chlorine redox A 1000 cm3 sample of water from a swimming pool which is being heavily chlorinated before use by the public required 15 cm 3 of iron(II) sulfate of concentration 0.6 mol l -1 to react completely. Calculate the number of moles of iron(II) sulfate used in the titration. Give your answer to three decimal places. .......................................... Q66: Calculate the concentration of chlorine in the swimming pool in mol l -1 . Give your answer to four decimal places. .......................................... Q67: Calculate the concentration of chlorine in the swimming pool in mg l -1 . Give your answer to one decimal place. .......................................... Q68: Present your working in a tidy fashion. An example is shown at the end. .......................................... .......................................... Key point The concentration of a reactant can be calculated from results of volumetric titrations. 4.10 Summary cloze test Summary exercise Online there is an interactive exercise summarising this topic. If you do not have access to the web, here is a paper version. Q69: The thermit reaction is used to generate molten iron, for example to weld railway lines in situ. The reactants are iron(iii) oxide and aluminium powder. The equation for the reaction is: a) Fe2 O3 + Al → Fe + Al2 O3 b) Fe2 O3 + 2Al → 2Fe + Al2 O3 c) 3FeO + 2Al →3 Fe + Al 2 O3 © H ERIOT-WATT U NIVERSITY Go online 160 TOPIC 4. OXIDISING OR REDUCING AGENTS .......................................... Q70: What word can be used to describe the oxide ion (O 2- )? a) Common b) Watching c) Spectator .......................................... Q71: The ionic equation can be written as: a) Fe + Al → Fe + Al b) Fe+ + Al → Fe + Al+ c) Fe3+ + Al → Fe + Al3+ .......................................... Q72: Oxidation is defined as . . . . . . of electrons. a) change b) loss c) gain .......................................... Q73: In this reaction what is oxidised? a) b) c) d) Al Fe Al3+ Fe3+ .......................................... Q74: What is acting as the oxidising agent? a) b) c) d) Al Fe Al3+ Fe3+ .......................................... Q75: Using the electrochemical series, which metal could not be used to reduce Fe 3+ ion to Fe? a) Copper b) Magnesium c) Manganese .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS Q76: You are required to make a mixture of aluminium (Al) and iron(III) oxide (Fe 2 O3 ) to use for this reaction. Use your data booklet to calculate how much Fe 2 O3 is required to add to 1 kg of Al to make a reaction mixture. a) 1 kg b) 2 kg c) 3 kg .......................................... 4.11 Summary Summary You should be able to state that: Elements as oxidising or reducing agents • A redox reaction is a reaction in which reduction and oxidation occur together, reduction being the gain of electrons by a reactant and oxidation being the loss of electrons by a reactant in a reaction. • An oxidising agent is a substance which accepts electrons. • A reducing agent is a substance which donates electrons. • Oxidising and reducing agents can be identified in redox reactions. • Elements with low electronegativities (metals) tend to form ions by losing electrons (oxidation) and so can act as reducing agents. • Elements with high electronegativities (non-metals) tend to form ions by gaining electrons (reduction) and so can act as oxidising agents. • The strongest reducing agents are found in Group 1. • The strongest oxidising agents come from Group 7. • The electrochemical series indicates the effectiveness of oxidising and reducing agents. Compounds as oxidising or reducing agents • Compounds can also act as oxidising or reducing agents. • Electrochemical series contain a number of ions and molecules. • The dichromate and permanganate ions are strong oxidising agents in acidic solutions whilst hydrogen peroxide is an example of a molecule which is a strong oxidising agent. • Carbon monoxide is an example of a gas that can be used as a reducing agent. © H ERIOT-WATT U NIVERSITY 161 162 TOPIC 4. OXIDISING OR REDUCING AGENTS Summary continued • Oxidising and reducing agents can be selected using an electrochemical series from a data booklet or can be identified in the equation showing a redox reaction. Use of oxidising agents • Oxidising agents are widely employed because of the effectiveness with which they can kill fungi and bacteria, and can inactivate viruses. • The oxidation process is also an effective means of breaking down coloured compounds making oxidising agents ideal for use as ‘bleach’ for clothes and hair. Ion-electron equations • Oxidation and reduction reactions can be represented by ion-electron equations. • When molecules or group ions are involved, if the reactant and product species are known, a balanced ion-electron equation can be written by adding appropriate numbers of water molecules, hydrogen ions and electrons. • Ion-electron equations can be combined to produce redox equations. Practical applications • Displacement reactions are example of redox reactions and oxidising and reducing agents can be identified in these and other redox reactions. • The technique of titration can be applied to redox reactions, allowing the concentration of a reactant to be calculated from results of volumetric titrations. 4.12 Resources Higher Chemistry for CfE, J Anderson, E Allan and J Harris, Hodder Gibson, ISBN 978-1444167528 © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS 4.13 163 End of topic test End of topic 4 test This end of topic test is available online. If you do not have access to the internet, here is a paper version. Q77: Which of the following reductions is the easiest? a) b) c) d) Cl2 + 2e- → 2Cl2H+ + 2e- → H2 Fe3+ + e- → Fe2+ Na+ + e- → Na .......................................... Q78: An oxidising agent is a substance which a) b) c) d) is oxidised in a reaction. accepts electrons. donates electrons. causes reduction. .......................................... Q79: Which of these is the equation for oxidation of bromide ions? a) b) c) d) 2Br- + 2e- → Br2 2Br- → Br2 + 2eBr2 + 2e- → 2BrBr2 → 2Br- + 2e.......................................... Q80: In which of these reactions is the reactant a reducing agent? a) b) c) d) Fe3+ + 3e- → Fe Fe2+ → Fe3+ + eH+ + HO- → H2 O 2H2 O + 2e- → H2 + 2OH.......................................... Q81: Which of these is the equation for the reduction of copper ion? a) b) c) d) Cu2+ + 2e- → Cu Cu2+ → Cu + 2eCu + 2e- → Cu2+ Cu → Cu2+ + 2e.......................................... © H ERIOT-WATT U NIVERSITY Go online 164 TOPIC 4. OXIDISING OR REDUCING AGENTS Q82: Consider the following two reactions. Cl2 + 2e- → 2ClI2 + 2e- → 2IWhich of these reactions will take place in solution? a) b) c) d) Chlorine will react with iodine. Iodide ions will react with chloride ions. Iodide ions will react with chlorine. Chloride ions will react with iodine. .......................................... Q83: The following reduction fits into the electrochemical series: standard reduction potentials at a value of E o = + 0.68 V. O2 + 2H+ + 2e- H2 O2 Which of these chemicals would convert H 2 O2 into O2 ? a) b) c) d) Sn4+ Fe2+ Sn2+ Fe3+ .......................................... Q84: How many electrons have to be added to balance this incomplete ion-electron equation? Hg2 Cl2 → 2Hg + 2Cla) b) c) d) Add 1 electron to the left hand side. Add 2 electrons to the right hand side. Add 2 electrons to the left hand side. Add 1 electron to the right hand side. .......................................... Q85: Consider the following two oxidations. Fe2+ → Fe3+ + eSn2+ → Sn4+ + 2eWhich of these reactions is likely to take place in solution? a) b) c) d) Tin(IV) ions will react with iron(II) ions. Tin(IV) ions will react with iron(III) ions. Iron(II) ions will react with tin(II) ions. Iron(III) ions will react with tin(II) ions. .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 4. OXIDISING OR REDUCING AGENTS Q86: In the equation below, highlight the chemical acting as a reducing agent. .......................................... Q87: In the equation below, highlight the chemicals which are necessary to act as an oxidising agent. .......................................... Q88: Which of the following contains the best oxidising agent? a) b) c) d) e) f) Cs+ (aq) Br- (aq) Ca (s) Cl2 (g) Fe2+ (aq) Sn (s) .......................................... Q89: Which of the following contains the best reducing agent? a) b) c) d) e) f) Cs+ (aq) Br- (aq) Ca (s) Cl2 (g) Fe2+ (aq) Sn (s) .......................................... Q90: Which of the following contains a substance which could be used as an oxidising agent or a reducing agent? a) b) c) d) e) f) Cs+ (aq) Br- (aq) Ca (s) Cl2 (g) Fe2+ (aq) Sn (s) .......................................... Look at this incomplete ion-electron equation. XeO3 → Xe Q91: How many water molecules have to be added to the product side? .......................................... © H ERIOT-WATT U NIVERSITY 165 166 TOPIC 4. OXIDISING OR REDUCING AGENTS Q92: How many hydrogen ions have to be added to the reactant side? .......................................... Q93: How many electrons have to be added to balance the charge? .......................................... Q94: On which side do the electrons get added? a) Reactant b) Product .......................................... Q95: The completed ion-electron equation is a) an oxidation. b) a reduction. .......................................... Hydrogen peroxide (H 2 O2 ) is used as a hospital antiseptic. A technician checking the concentration of peroxide titrated 25.0 cm 3 portions against 0.125 mol l-1 potassium permanganate and found an average titration value of 12.8 cm 3 . The redox reaction is 2MnO 4- + 6H+ + 5H2 O2 → 2Mn2+ + 8H2 O + 5O2 Q96: Calculate the number of moles of potassium permanganate used in the titration. Give your answer to 4 decimal places. .......................................... Q97: What ratio of permanganate to hydrogen peroxide should be used in the calculation of the concentration of the peroxide solution? a) b) c) d) 1:2.5 1:5 1:1 2:1 .......................................... Q98: Calculate the concentration of the hydrogen peroxide solution in mol l -1 to 2 decimal places. .......................................... Q99: Calculate the concentration of H 2 O2 in g l-1 . Give your answer to 2 decimal places. .......................................... .......................................... © H ERIOT-WATT U NIVERSITY 167 Topic 5 Chromatography Contents 5.1 Prior knowledge . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 169 5.2 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.3 Paper chromatography . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 169 172 5.3.1 Thin layer chromatography . . . . . . . . . . . . . . . . . . . . . . . . . 5.3.2 Chromatography of protein hydrolysates . . . . . . . . . . . . . . . . . . 174 175 5.4 Gas-liquid chromatography . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.5 Size-exclusion chromatography . . . . . . . . . . . . . . . . . . . . . . . . . . . 178 180 5.6 Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.7 Resources . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 183 183 5.8 End of topic test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 184 168 TOPIC 5. CHROMATOGRAPHY Prerequisite knowledge You should already know that: • chemical analysis permeates all aspects of chemistry. It is important that you understand the significance of analysis and are aware of how to carry out simple analytical techniques (National 4, Unit 3); • analytical techniques could include: ◦ chromatography; ◦ flame tests; ◦ pH measurement using indicators / pH meters; ◦ separation techniques (National 4, Unit 3); • titration is an analytical technique used to determine the accurate volumes involved in chemical reactions such as neutralisation (National 5, Unit 1); • an indicator is used to show the end-point of the reaction (National 5, Unit 1); • chemists play an important role in society by monitoring our environment to ensure that it remains healthy and safe and that pollution is tackled as it arises (National 5, Unit 3); • a variety of methods exist which enable chemists to monitor the environment both qualitatively and quantitatively, such as acid/base titration, precipitation and flame testing (National 5, Unit 3). Learning objectives By the end of this topic you should be able to state that: • in chromatography, differences in the polarity / size of molecules are exploited to separate the components present within a mixture; • depending on the type of chromatography in use, the identity of a component can be indicated either by the distance it has travelled or by the time it has taken to travel through the apparatus (retention time); • the results of a chromatography experiment can sometimes be presented graphically showing an indication of the quantity of substance present on the yaxis and retention time on the x-axis. Note: Learners are not required to know the details of any specific chromatographic method or experiment. © H ERIOT-WATT U NIVERSITY TOPIC 5. CHROMATOGRAPHY 5.1 169 Prior knowledge Test your prior knowledge Q1: Which of the following techniques would you use to separate a mixture of water and alcohol? a) b) c) d) Distillation Filtration Chromatography Titration .......................................... Q2: Which of the following techniques would you use to separate a mixture of water and sand? a) b) c) d) Distillation Filtration Chromatography Titration .......................................... Q3: A precipitation reaction is one where: a) b) c) d) the pH of a reaction moves closer to 7. two solutions react to form a solid. two small molecules react to produce a larger molecule and water. a metal higher up in the electrochemical series displaces one lower down from its ions in solution. .......................................... 5.2 Introduction Much of chemistry is concerned with producing materials. These could be either a small amount of a novel substance produced in a laboratory to enable further research into new, unknown compounds or, alternatively, the manufacture of a large quantity of an industrial product. The other main strand of chemistry is concerned with analysis; finding out the structure or composition of a natural or artificial material. Chemical analysis has importance in: • environmental protection; • chemical manufacturing, e.g. pharmaceuticals; • consumer protection, e.g. food analysis; • forensics; © H ERIOT-WATT U NIVERSITY Go online 170 TOPIC 5. CHROMATOGRAPHY • bioanalysis and clinical chemistry; • experimental design, including controlling chemical processes. Analytical methods that are widely used to separate and identify the components of mixtures come under the heading of chromatography. There are several different types of chromatography, but the name derives from the early methods used by the Russian, Michael Tswett, to separate mixtures of plant pigments into a pattern of coloured components. The Greek words chroma (colour) and graphein (to write) were chosen to name the method. All chromatographic methods involve a mobile phase moving over a stationary phase. Separation of the components in the sample occurs because they have different solubilities (strictly, partition coefficients) between the stationary and mobile phases. Substances present in the initial mixture which dissolve more in the stationary phase will move more slowly than materials which dissolve more in the mobile phase. Hornets versus bees Go online To illustrate this separation process, follow the images below of some bees and hornets moving over a flower bed. The bees spend some time collecting nectar from the (stationary) flowers, and so arrive at the edge of the bed later than the hornets. Hornets versus bees - start © H ERIOT-WATT U NIVERSITY TOPIC 5. CHROMATOGRAPHY The bees land on the flowers to collect nectar The hornets finish first The bees and hornets are separated .......................................... © H ERIOT-WATT U NIVERSITY 171 172 TOPIC 5. CHROMATOGRAPHY 5.3 Paper chromatography In paper chromatography the mixture of components to be separated is placed as a small spot close to the bottom of a rectangular piece of absorbent paper (like filter paper). The bottom of the paper is placed in a shallow pool of solvent in a tank. An example of the solvent would be an alcohol. Owing to capillary attraction, the solvent is drawn up the paper, becoming the mobile phase. The solvent front is clearly visible as the chromatography progresses. When the paper is removed from the solvent, the various components in the initial spot have moved different distances up the paper. Chromatography of blue and black inks The start and final positions of a chromatographic analysis are shown below. Go online Start of chromatographic analysis End of chromatographic analysis This separation depends on the different polarities of the various components in the sample. The components are dissolved in the moving solvent and the water trapped in the paper. Substances which are less polar will dissolve mainly in the solvent mobile phase and so will move further up the paper than more polar substances which dissolve more in the aqueous stationary phase. © H ERIOT-WATT U NIVERSITY TOPIC 5. CHROMATOGRAPHY Q4: By observing the chromatography simulation above, which ink (blue or black) has the most components? a) Blue b) Black c) Both the same. .......................................... Q5: Which material in the black ink has stayed longest on the stationary phase? a) b) c) d) e) Red Yellow Dark blue Blue Green .......................................... Q6: What can be deduced about the polarity of the green component in black ink? a) It is more polar than the red component. b) It is less polar than the red component. c) It is a non-polar substance. .......................................... Q7: The red coloured spot has moved furthest, this would indicate that the red material: a) has the highest solubility in the solvent. b) has the lowest solubility in the solvent. c) has the lowest molecular mass. .......................................... Q8: The movement of materials on paper chromatography is often described by an R f (relative to the front) value, which is the distance travelled by the spot divided by the distance travelled by the solvent front. As long as the conditions of chromatography remain the same, a compound will have a constant R f value. Which colour in the black ink could have an R f value of 0.4? a) b) c) d) e) Red Yellow Dark blue Blue Green .......................................... Q9: Both the blue and dark blue spots from both the original inks have moved similar distances. What might you conclude from this? .......................................... © H ERIOT-WATT U NIVERSITY 173 174 TOPIC 5. CHROMATOGRAPHY Q10: You set up a paper chromatography experiment using butanol as the mobile phase. All the dyes in your ink hardly move from the initial position; all have low R f values. What does this tell you about your choice of mobile phase? What could you do about it? .......................................... 5.3.1 Thin layer chromatography Although paper chromatography is still used today, it has been largely replaced by thin layer chromatography (TLC). In this method, a support plate of glass or aluminium is coated, usually with a thin layer of silica, alumina or cellulose. The processing is identical to paper chromatography but TLC allows a more rapid separation (which prevents the spots spreading too far) and makes detection of the spots easier. Most materials are not coloured, but can still be separated by chromatography. An additional stage is required with non-visible components. The invisible spots on paper or thin layer chromatography are revealed by use of a locating reagent. These are applied once the chromatography is complete and react with the compounds in the spots to produce a coloured derivative. For example, ninhydrin solution can be sprayed onto chromatograms to reveal amino-acids as purple spots. Separation of black ink on a TLC plate (https:/ / commons.w ikimed ia.or g/ w iki/ F ile:T LC_black_ink.jpg by http:/ / en.w iki ped ia.or g/ w iki/ User :Natr ij is licensed under http:/ / cr eativecommons.or g/ license s/ by-sa/ 3.0/ ) © H ERIOT-WATT U NIVERSITY TOPIC 5. CHROMATOGRAPHY 175 Further developments In another TLC detection system, the silica stationary phase is mixed with a fluorescent dye, so that at the end of the process, viewing the plate under an ultraviolet lamp will cause the background to glow (often an eerie green) except where there are spots, which appear black. TLC is a rapid method for indicating qualitatively what materials are present in a sample mixture. When carrying out an experimental synthesis, for example, putting a small spot of your reaction mixture onto a TLC plate and developing it will show you whether your reactants have turned into products. It is not so effective at giving quantitative information about how much of a certain material is in the sample. To obtain this information different systems are employed. 5.3.2 Chromatography of protein hydrolysates Chromatography of protein hydrolysates The diagrams below show the results of a simulated experiment on the chromatography of protein hydrolysates. These results show the chromatographic separation of the amino acids present in a section of protein. Three different proteins X, Y and Z have been hydrolysed. Protein X results © H ERIOT-WATT U NIVERSITY Go online 176 TOPIC 5. CHROMATOGRAPHY Protein Y results Protein Z results Q11: Look at the diagram showing the results of a chromatographic separation for the hydrolysed protein X. How many amino acids are present ? .......................................... Q12: Name any amino acid not present in X. .......................................... Q13: It is possible to conclude from the results that hydrolysed protein X contains: a) b) c) d) B and C A and B A, B and C A, B and D © H ERIOT-WATT U NIVERSITY TOPIC 5. CHROMATOGRAPHY .......................................... Q14: Referring to protein sample X, draw your prediction of the chromatogram which would result if this protein section was hydrolysed and separated alongside samples of A, B, C and D. .......................................... Q15: Look at the diagram showing the results of a chromatographic separation for the hydrolysed protein Y. Explain fully your conclusions about the composition of Y from the results of the experiment. There could be three marks for this answer in an exam setting. Try to give three pieces of information. Write an answer before revealing the possible model answer. .......................................... Q16: Look at the diagram showing the results of a chromatographic separation for the hydrolysed protein Z. How many amino acids are present in the hydrolysed protein Z ? .......................................... Q17: Name an amino acid which is not present in Z. .......................................... Q18: Which amino acid seems to be present in the greatest amount? .......................................... Q19: Look at the structures of the four standard amino acids used in this simulation and the position of these after chromatography. Decide whether the following statement is true or false: • The separation in this chromatography depends on molecular mass. a) True b) False .......................................... Q20: Give a piece of evidence to support your answer to the previous question. .......................................... © H ERIOT-WATT U NIVERSITY 177 178 TOPIC 5. CHROMATOGRAPHY Q21: If you examined the products of hydrolysis of a complex protein, say the enzyme pepsin, what do you think the chromatogram would look like? .......................................... Q22: How might you suggest this problem could be resolved? .......................................... 5.4 Gas-liquid chromatography In gas-liquid chromatography (GLC) the stationary phase is a high boiling point liquid held on an inert, finely-powdered support material, and the mobile phase is a gas (often called the carrier gas). The stationary phase is packed into a tubular column usually of glass or metal, with a length of 1 to 3 metres and internal diameter about 2 mm. One end of the column is connected to a gas supply (often nitrogen or helium) via a device which enables a small volume of liquid sample, containing the mixture to be analysed, to be injected into the gas stream. The other end of the column is connected to a device which can detect the presence of compounds in the gas stream. The column is housed in an oven to enable the temperature to be controlled throughout the chromatographic analysis. One reason for this is that the materials to be analysed must be gases during the analysis, so that gas-liquid chromatography is often carried out at elevated temperatures. GLC apparatus A mixture of the material to be analysed is injected into the gas stream at zero time. The individual components travel through the packed column at rates which depend on their partition coefficients between the liquid stationary phase and the gaseous mobile © H ERIOT-WATT U NIVERSITY TOPIC 5. CHROMATOGRAPHY phase. The detector is set to measure some change in the carrier gas that signals the presence of material coming from the end of the column. Some detectors measure the thermal conductivity of the gas, others burn the material from the column in a hydrogen-air flame and measure the presence of ions in the flame. The signal from the detector is recorded and plotted against time to give a series of peaks each with an individual retention time. A typical trace, chromatogram, for a complex mixture, premium grade petrol, is shown below. Chromatogram of premium grade petrol As long as the conditions remain constant (same column and stationary phase, same pressure of gas, and same temperature) the retention time is the same for a given compound. By chromatographing known materials (standards) the identity of many peaks in a GLC trace can be established. The other important feature of many chromatograms is that the area under the peak is directly proportional to the amount of material present. The composition of petrol is changed with the seasons by the suppliers. In the cold winter months petrol needs a higher proportion of more volatile, low-boiling components; in summer too much of this composition would evaporate, so more highboiling compounds are present. GLC analysis is used by the oil companies to ensure consumers are getting the correct grade of petrol. © H ERIOT-WATT U NIVERSITY 179 180 TOPIC 5. CHROMATOGRAPHY Gas-liquid chromatography Go online Q23: The retention times, under the same conditions as the chromatogram figure above, for four compounds are shown in the table below: Hydrocarbon Structure Retention time Butane C4 H10 5.5 min Pentane C5 H12 7.4 min Benzene C6 H6 12.7 min Toluene C7 H8 15.1 min Which of these hydrocarbons is present in the greatest amount in petrol? a) b) c) d) Butane Pentane Benzene Toluene .......................................... Q24: How are retention time and molecular mass related for these four hydrocarbons? a) The retention time increases as mass increases. b) The retention time decreases as mass increases. c) They are not related. .......................................... Q25: Using the answer to the previous question, suggest a possible hydrocarbon for the peak with retention time 20.8 min. a) b) c) d) Hexane (C6 H14 ) Heptane (C7 H16 ) Xylene (C8 H10 ) Propane (C3 H8 ) .......................................... 5.5 Size-exclusion chromatography The previous separations depended mainly on the differences in polarities between the components in a sample. Size-exclusion chromatography, sometimes called gel filtration, separates on the basis of molecular size. It is widely used to characterise polymers, including biological material. A column is packed with porous beads and the sample containing a range of differently sized molecules added to the top in a suitable solvent. A flow of solvent then moves down the column. The sample components are detected as they leave the column and © H ERIOT-WATT U NIVERSITY TOPIC 5. CHROMATOGRAPHY 181 a graph of detector response against time is constructed to reveal the peaks of eluted compounds. There is a range of materials with different pore sizes in the beads, so that a wide range of sizes of analytes (materials to be separated) can be chromatographed. In order to quantify the sizes of analytes there are standards containing molecules of a defined range of sizes which can be used in the system. Only the smaller molecules can enter the pores in the stationary beads; the larger molecules are excluded and remain in the mobile phase solvent. You can see this in the diagram below. Gel filtration Go online Q26: Larger molecules in the sample will leave the column (be eluted) first. Why does this happen? a) The smaller molecules adhere more strongly to the beads. b) The larger molecules are excluded form the pores in the stationary beads. c) The larger molecules are heavier and move to the bottom faster. .......................................... © H ERIOT-WATT U NIVERSITY 182 TOPIC 5. CHROMATOGRAPHY Q27: If the pores in the beads were too small to allow any solute molecules to enter the beads, what would happen? a) The sample molecules would remain on the top of the column and never be eluted. b) The sample molecules would be slowly eluted from the column in many peaks. c) The sample molecules would elute from the column in a single peak. .......................................... Q28: A size-exclusion column was constructed and a standard containing three proteins A, B and C with molecular masses of 1,000, 3,000 and 6,000 respectively was applied to the column and eluted with solvent. The pattern of elution is shown below. Proteins A, B and C Starting with the earliest, what is the order of elution? a) b) c) d) A, B then C A, C then B C, B then A B, A then C .......................................... Q29: A sample, X, containing unknown proteins was applied to the same column and eluted under identical conditions to the previous question. The pattern is shown below. Unknown proteins What can you tell about the proteins in sample X? .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 5. CHROMATOGRAPHY Q30: Think very carefully. Is the answer to the question above absolutely correct? .......................................... Q31: Another experiment was performed on this system by mixing the standard mixture and the unknown sample then eluting this mixture. What would you expect the elution pattern to look like? .......................................... Q32: What could you do to try and establish whether there was more than one type of protein with the same size in the sample? .......................................... .......................................... 5.6 Summary Summary You should now be able to state that: • in chromatography, differences in the polarity / size of molecules are exploited to separate the components present within a mixture; • depending on the type of chromatography in use, the identity of a component can be indicated either by the distance it has travelled or by the time it has taken to travel through the apparatus (retention time); • the results of a chromatography experiment can sometimes be presented graphically showing an indication of the quantity of substance present on the y-axis and retention time on the x-axis. Note: Learners are not required to know the details of any specific chromatographic method or experiment. 5.7 • Resources Higher Chemistry for CfE, J Anderson, E Allan and J Harris, Hodder Gibson, ISBN 978-1444167528 © H ERIOT-WATT U NIVERSITY 183 184 TOPIC 5. CHROMATOGRAPHY 5.8 End of topic test End of topic 6 test Go online Q33: This question refers to the chromatographic analysis of hydrolysed protein section P. Name the amino acids which are present in the hydrolysed protein section P. .......................................... Q34: How many amino acids are present in hydrolysed protein section P? .......................................... Q35: From the results, it can be concluded that the hydrolysed protein section P contains: a) b) c) d) A and B A and B only A, B and C A, B, C and D .......................................... Q36: Which of these protein sections could be P? a) © H ERIOT-WATT U NIVERSITY TOPIC 5. CHROMATOGRAPHY b) c) d) .......................................... Q37: If protein section Q, shown below, were hydrolysed and run against A, B, C and D, which spots would be present? a) b) c) d) A only A and D A, C and D A, B, C and D .......................................... © H ERIOT-WATT U NIVERSITY 185 186 TOPIC 5. CHROMATOGRAPHY Q38: Gas chromatograms of the hydrocarbons found in two sediment samples a few miles from an oil production platform are shown below. The numbers refer to the number of carbon atoms in the straight chain n-alkanes. Pr and Ph refer to pristane and phytane, two terpenoid hydrocarbons. Gas chromatography separates volatile compounds in order of boiling point. Pristane and phytane are highly branched hydrocarbons containing 19 and 20 carbon atoms respectively. Looking at the chromatograms, which of the following statements is correct? a) b) c) d) You can tell nothing about boiling points from these chromatograms. Boiling points of hydrocarbons with the same number of carbon atoms are the same. All hydrocarbons are present in these samples. Branched alkanes have lower boiling points than n-alkanes with the same number of carbon atoms. © H ERIOT-WATT U NIVERSITY TOPIC 5. CHROMATOGRAPHY .......................................... Q39: Samples of hydrocarbons which have been derived from crude oil spills and those from natural plant waxes differ in their carbon preference index (CPI). Plant waxes contain a preponderance of hydrocarbons with odd numbers of carbon atoms over those with even numbers (CPI > 1); crude oil hydrocarbons show no such pattern (CPI = 1). Look particularly at the hydrocarbons from 27 to 36. What do you think was the source of the sample? a) Plant waxes b) Cannot tell c) Crude oil .......................................... Q40: Sample A was taken from a place where marine currents supply new material on a regular basis. Sample B was from a region where hydrocarbon breakdown is more rapid than addition of new material. Comparing these two samples which of the following statements is correct? a) All hydrocarbons degrade at the same rate. b) Alkanes with odd numbers of carbon atoms are degraded faster than those with even numbers. c) Shorter hydrocarbons are degraded more slowly than longer ones. d) Branched alkanes, such as pristane and phytane, are degraded more slowly than n-alkanes. .......................................... Q41: What happens to hydrocarbons when they are degraded in the environment? .......................................... Q42: From your knowledge of chemistry describe why marine oil spills are frequently treated with detergents/emulsifiers in attempts to clear them more rapidly. .......................................... .......................................... © H ERIOT-WATT U NIVERSITY 187 188 TOPIC 5. CHROMATOGRAPHY © H ERIOT-WATT U NIVERSITY 189 Topic 6 Volumetric analysis Contents 6.1 Prior knowledge . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 191 6.2 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.2.1 Titration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 192 193 6.3 Quality control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.4 Practical applications of titrations . . . . . . . . . . . . . . . . . . . . . . . . . . 194 194 6.4.1 The purity of aspirin tablets . . . . . . . . . . . . . . . . . . . . . . . . . 6.4.2 Antacid tablet CaCO3 content (back titration) . . . . . . . . . . . . . . . 194 195 6.4.3 The chloride ion content of seawater (precipitation) . . . . . . . . . . . . 6.5 Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 196 198 6.6 Resources . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.7 End of topic test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 198 199 190 TOPIC 6. VOLUMETRIC ANALYSIS Prerequisite knowledge Before you begin this topic, you should already know that: • a neutralisation reaction is one in which an acid reacts with a base to form water. A salt is also formed in this reaction (National 4, Unit 1); • titration is an analytical technique used to determine the accurate volumes involved in chemical reactions such as neutralisation (National 5, Unit 1); • an indicator is used to show the end-point of the reaction (National 5, Unit 1); • combinations of oxidation and reduction half reactions form redox equations (Higher, Unit 3, Topic 5); • the technique of titration can be applied to redox reactions, allowing the concentration of a reactant to be calculated from results of volumetric titrations (Higher, Unit 3, Topic 5). Learning objectives By the end of this topic you should be able to state that: • volumetric analysis involves using a solution of accurately known concentration in a quantitative reaction to determine the concentration of another substance; • a solution of accurately known concentration is known as a standard solution; • the volume of reactant solution required to complete the reaction is determined by titration; • calculations from balanced equations can then be carried out to calculate the concentration of the unknown solution; • redox titrations are based on redox reactions; • substances such as potassium permanganate(VII), which can act as their own indicators, are very useful reactants in redox titrations; • the concentration of a substance can be calculated from experimental results by use of a balanced equation. © H ERIOT-WATT U NIVERSITY TOPIC 6. VOLUMETRIC ANALYSIS 6.1 191 Prior knowledge Test your prior knowledge Q1: What is the name of the technique which separates substances by their solubilities? a) b) c) d) Chromatography Distillation Titration Precipitation .......................................... Q2: What is the term given to when a titration has just been neutralised and no more? a) b) c) d) End point Titre Neutralisation Burette .......................................... Q3: Reaction of an acid and an alkali will produce which products? a) b) c) d) A salt and hydrogen A salt and water A salt, water and carbon dioxide A salt and oxygen .......................................... © H ERIOT-WATT U NIVERSITY Go online 192 TOPIC 6. VOLUMETRIC ANALYSIS 6.2 Introduction You should be familiar with volumetric analysis as a means of obtaining an accurate concentration of a substance in solution. A solution of accurately known concentration, a standard solution, is used to titrate the unknown solution until an end-point is obtained. Calculation then enables the unknown concentration to be found. Volumetric analysis equipment Go online The diagram below shows the equipment that you would use to carry out volumetric analysis. Q4: a) b) c) d) e) Identify the burette from the diagram above. A B C D E .......................................... Q5: a) b) c) d) e) Identify the pipette from the diagram above. A B C D E .......................................... Q6: a) b) c) d) e) Identify the indicator from the diagram above. A B C D E © H ERIOT-WATT U NIVERSITY TOPIC 6. VOLUMETRIC ANALYSIS 193 .......................................... Q7: Identify the volumetric flask from the diagram above. a) b) c) d) e) A B C D E .......................................... 6.2.1 Titration The following example describes an acid-based titration. A metal plating plant produces waste nitric acid which is discharged into a holding tank before disposal. The process chemists need to know the concentration of the acid in the tank, so take regular samples to estimate the nitric acid concentration by titration with standard sodium hydroxide solution. A 25 ml sample from the tank was titrated with 0.100 mol -1 NaOH and required 28.5 ml for complete neutralisation. What is the concentration of nitric acid in mol -1 and g -1 ? 1. Write a balanced equation: HNO 3 + NaOH → NaNO 3 + H2 O The mol relationship: 1 mol HNO 3 reacts with 1 mol NaOH 2. Moles of NaOH used = concentration × volume = 0.100 × 0.0285 = 0.00285 mol 3. Moles of HNO3 in sample = 0.00285 Concentration = moles/volume = 0.00285/0.025 = 0.114 mol -1 4. Molecular mass of HNO3 = 1 + 14 + 3 × 16 = 63 (g mol -1 ) 0.114 mol -1 HNO3 is 0.114 × 63 = 7.182 g -1 Titration Q8: A soap factory produces an alkaline waste containing potassium hydroxide solution. A 25 ml sample from the waste was titrated with 0.150 mol -1 hydrochloric acid and required 34.5 ml for complete neutralisation. What is the concentration of potassium hydroxide in mol -1 and g -1 ? .......................................... © H ERIOT-WATT U NIVERSITY Go online 194 TOPIC 6. VOLUMETRIC ANALYSIS 6.3 Quality control Quality assurance is often applied to an end-product or service. The consumer should be able to be assured that whatever is being supplied will meet certain defined criteria. Quality control is used internally by manufacturers, say a pharmaceutical manufacturer, to assess the progress of the production. At various critical stages, samples are taken and analysed to ensure that "all is well" with the process and to correct any problems before they lead to further difficulties. Quality control Go online Q9: An antiseptic healing cream used for mild skin irritation such as nappy rash, sunburn or abrasions is made by taking a slurry of zinc oxide (ZnO) in water and blending this with oils and waxes to make an emollient cream. Before this blending, the zinc oxide suspension is sampled and titrated with standard hydrochloric acid to determine the concentration of zinc oxide. It should contain between 400 and 450 g ZnO -1 of slurry. The sample volume of 1.00 ml is neutralised completely by 20.64 ml of 0.500 mol -1 hydrochloric acid. Is the batch within the requirements? .......................................... 6.4 Practical applications of titrations You have already met redox titrations in a previous topic. The following pages contain some useful titration experiments for you to try. There are suggested results, so that you can practise the calculations. 6.4.1 The purity of aspirin tablets The purity of aspirin tablets The chemical structure of the analgesic aspirin (acetylsalicylic acid) is shown below. Go online Aspirin Q10: Look at the chemical structure. What method would you suggest to quantify the mass of aspirin in a tablet? © H ERIOT-WATT U NIVERSITY TOPIC 6. VOLUMETRIC ANALYSIS .......................................... Q11: Each aspirin tablet should have 300 mg of acetylsalicylic acid. Calculate the relative formula mass of aspirin and then calculate how many moles of aspirin should be in one tablet. .......................................... Q12: A sample tablet of aspirin was dissolved in water and titrated with 0.300 mol -1 sodium hydroxide solution, using phenolphthalein as indicator. If the tablet was 100% aspirin, what volume of sodium hydroxide solution would be required? .......................................... Q13: As a method for checking the aspirin content of tablets this experiment could be improved in several ways. What might you suggest? .......................................... 6.4.2 Antacid tablet CaCO3 content (back titration) Many indigestion remedies contain 'antacids', compounds which react with the hydrochloric acid in stomach juices and so reduce the excess acidity. One of the commonest of these is calcium carbonate. Antacid tablets (Antacid -L47 8 by http:/ / commons.w ikimed ia.or g/ w iki/ User :Mid nightcomm is licensed under http:/ / cr eativecommons.or g/ licenses/ by-sa/ 3.0/ ) You cannot estimate the amount of antacid in an indigestion tablet directly because the material is insoluble in water. Consequently, you estimate the calcium carbonate content of antacid tablets by performing a "back titration". A tablet is added to a known volume of standard hydrochloric acid and the acid remaining is measured by titration with standardised sodium hydroxide solution. From this figure, the amount of acid neutralised by the tablet can be calculated. The method has the added advantage that you don't need to know the composition of the tablet to obtain a value for its effectiveness. © H ERIOT-WATT U NIVERSITY 195 196 TOPIC 6. VOLUMETRIC ANALYSIS Method of back titration An antacid tablet is placed in a conical flask with about 25 ml of water and 5.00 ml of 5.00 mol -1 hydrochloric acid is added. The tablet effervesces and the solution is gently boiled for a minute to ensure complete reaction. Any cloudiness remaining will be due to other ingredients, e.g. starch, in the tablet. The solution is then cooled, a few drops of indicator solution, phenolphthalein, is added and the solution titrated with 0.500 mol -1 sodium hydroxide solution, until a faint pink colour remains. Back titration Go online Q14: Assuming the volume of sodium hydroxide solution needed to neutralise the remaining HCl was 22.0 ml, what mass of calcium carbonate is in one tablet? Try this calculation yourself before looking at the answer. .......................................... 6.4.3 The chloride ion content of seawater (precipitation) The salinity (saltiness) of sea water is very important in determining which animals and plants can survive there and in driving the major ocean currents. The density of sea water depends on its salinity (the more saline the denser) and temperature (higher temperatures expand water, making it less dense). Gulf stream You may have heard of the Gulf Stream, see image above, which is a surface water current that carries warm water from the tropics north-east across the Atlantic to bathe western Scotland and allow palm trees to grow in Plockton! It is part of a circulation that involves this warm surface water cooling in the Northern hemisphere, becoming more dense and sinking to form a return sub-sea current flowing south-west. There is some worry that, as the Earth generally becomes warmer, the Gulf Stream that keeps Britain warm might stop. © H ERIOT-WATT U NIVERSITY TOPIC 6. VOLUMETRIC ANALYSIS 197 Since the predominant salt in seawater is sodium chloride, a good estimate of salinity can be obtained by determining the chloride ion content. The chloride ion content can be calculated by titrating the seawater with standard silver nitrate solution until excess silver ions can be seen. This is done by adding potassium chromate(VI). In solution chromate(VI) ion is yellow, but with silver ion it forms an intense brick red precipitate. The vast amount of chloride ion in seawater is thought to have originated from the 'outgassing' of the mantle as volcanos in the very early history of the Earth. The negative ions that are carried in rivers due to weather erosion of rocks contain very little chloride ion; they are mainly hydrogencarbonate (HCO 3 - ) owing to the action of carbonic acid (H2 CO3 ) on rock minerals. Any chloride ion present has probably been recycled from rain produced when clouds form over the salty oceans. The chloride ion content of seawater (precipitation) Q15: Seven ions constitute more than 99% of the ions in seawater and the ratios of these ions is constant throughout the world's oceans, so estimating one (e.g. chloride) can lead to estimates of the others and hence overall salinity. Which seven ions do you think are the major ones in seawater? .......................................... Q16: A 10 ml sample of seawater is carefully measured into a conical flask and diluted with about 20 ml of water. A few drops of a solution of potassium chromate(VI), KCrO 4 , is added as indicator. The solution is titrated with a standard solution of silver nitrate, AgNO3, containing 0.50 mol -1 , until a trace of red precipitate remains. What do you think the red precipitate might be? .......................................... Q17: Assuming the volume of silver nitrate solution needed to react with the chloride ion in seawater was 12.0 ml, what is the salinity of seawater in g NaCl -1 ? Try this calculation before looking at the answer. .......................................... © H ERIOT-WATT U NIVERSITY Go online 198 TOPIC 6. VOLUMETRIC ANALYSIS .......................................... 6.5 Summary Summary You should now be able to state that: 6.6 • • volumetric analysis involves using a solution of accurately known concentration in a quantitative reaction to determine the concentration of another substance; • a solution of accurately known concentration is known as a standard solution; • the volume of reactant solution required to complete the reaction is determined by titration; • calculations from balanced equations can then be carried out to calculate the concentration of the unknown solution; • redox titrations are based on redox reactions; • substances such as potassium permanganate(VII), which can act as their own indicators, are very useful reactants in redox titrations; • the concentration of a substance can be calculated from experimental results by use of a balanced equation; • quality control of chemical processes requires analysis to ensure that the process requirements are being met. Resources Higher Chemistry for CfE, J Anderson, E Allan and J Harris, Hodder Gibson, ISBN 978-1444167528 © H ERIOT-WATT U NIVERSITY TOPIC 6. VOLUMETRIC ANALYSIS 6.7 199 End of topic test End of Topic 6 test Q18: As vegetable oils age they produce fatty acids. It is, therefore, important to be able to estimate the concentration of 'free fatty acids' in samples of vegetable oils. This is usually done by an acid-base titration. A sample of the oil is titrated with standard potassium hydroxide solution. Explain why the oil cannot be titrated directly with aqueous potassium hydroxide. .......................................... Q19: The acid-based titration reaction is carried out in a solvent mixture, often a mixture of ethanol and ether, which dissolves both vegetable oils and aqueous potassium hydroxide. An example procedure is as follows: 1. A sample of the oil weighing 5.53 g is dissolved in about 50 ml of the solvent in a 250 ml conical flask. 2. A few drops of indicator solution (phenolphthalein) are added. 3. The solution is titrated with 0.1 mol l -1 potassium hydroxide solution until a faint pink colour persists, indicating the end-point. 4. The titration value for this sample is 12.32 ml. Calculate the mass, in grammes, of oleic acid (GFM = 282) in the flask. .......................................... Q20: Calculate the percentage of oleic acid in the oil sample. .......................................... Q21: When making biodiesel from vegetable oil it important to start with a neutral oil, so any free fatty acid is neutralised with sodium hydroxide. What mass of solid sodium hydroxide, in kg, would need to be added to 100 kg of this oil sample to neutralise it exactly? .......................................... Q22: The water in swimming pools can be kept sterile by adding chlorine, which kills micro-organisms. Chlorine levels in swimming pools can be monitored by titration with iron(II) sulfate. The redox equation for this is: 2Fe2+ + Cl2 → 2Fe3+ + 2ClA 1000 cm3 sample of water from a swimming pool which is being heavily chlorinated before use by the public required 15 cm3 of iron(II) sulfate of concentration 0.6 mol l-1 to react completely. Calculate the number of moles of iron(II) sulfate used in the titration. Give your answer to three decimal places. .......................................... © H ERIOT-WATT U NIVERSITY Go online 200 TOPIC 6. VOLUMETRIC ANALYSIS Q23: Calculate the concentration of chlorine in the swimming pool in mol l -1 . Give your answer to four decimal places. .......................................... Q24: Calculate the concentration of chlorine in the swimming pool in mg l -1 . Give your answer to one decimal place. .......................................... © H ERIOT-WATT U NIVERSITY 201 Topic 7 End of unit test 202 TOPIC 7. END OF UNIT TEST End of unit 3 test Q1: Go online On going down a group in the Periodic Table, the first ionisation energy: a) increases. b) decreases. .......................................... Q2: a) b) c) d) When the enthalpy change has a positive sign, the reaction is: exothermic. endothermic. large. small. .......................................... Q3: Ionic bonding is most likely when the electronegativity difference between the elements is: a) b) c) d) exothermic. endothermic. large. small. .......................................... Q4: The same reaction was carried out at four different temperatures. The table below shows the time taken for the reaction to occur. Temp (◦ C) 20 30 40 50 Time (seconds) 60 30 14 5 The results show that: a) b) c) d) a small rise in temperature results in a large increase in reaction rate. the reaction is endothermic. the activation energy increases with increasing temperature. the rate of the reaction is directly proportional to the temperature. .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 7. END OF UNIT TEST Q5: The potential energy diagram below refers to the reversible reaction involving reactants R and products P. What is the value of the activation energy for the forward reaction (reactants to products)? a) b) c) d) 10 kJ mol-1 20 kJ mol-1 40 kJ mol-1 60 kJ mol-1 .......................................... Q6: What is the enthalpy change for the forward reaction (reactants to products)? a) b) c) d) -30 kJ mol-1 -20 kJ mol-1 +20 kJ mol-1 +30 kJ mol-1 .......................................... Q7: Which of the following molecules may be described as polar? a) b) c) d) .......................................... © H ERIOT-WATT U NIVERSITY 203 204 TOPIC 7. END OF UNIT TEST Q8: The melting points of Group 7 elements increase on descending the group because the . . . . . . . . . . . . . . . . . . increase. a) b) c) d) mean bond enthalpies nuclear charges covalent bond lengths London dispersion forces .......................................... Q9: The difference between the covalent radii of lithium and carbon is mainly due to the difference in the: a) b) c) d) number of neutrons. mass of each atom. number of electrons. number of protons. .......................................... Q10: In general, covalent substances have lower melting points than ionic substances because: a) b) c) d) covalent bonds have no electrostatic attractive forces. covalent compounds are composed of non-metals which have low melting points. bonds between molecules are weaker than bonds between ions. ionic bonds are stronger than covalent bonds. .......................................... Q11: The product of the oxidation of the above compound is: a) b) c) d) 4-methylpentan-2-one. 2-methylpentanal. 2-methylpentan-4-one. 4-methylpentanal. .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 7. END OF UNIT TEST Q12: Rum flavouring is based on the compound with the formula shown above. It can be made from: a) b) c) d) propanol and ethanoic acid. ethanol and butanoic acid. butanol and methanoic acid. ethanol and propanoic acid. .......................................... Q13: What product(s) would be expected upon dehydration of the alcohol shown above? a) b) c) d) 2-methylbut-2-ene only 2-methylbut-2-ene and 2-methylbut-1-ene 3-methylbut-1-ene and 2-methylbut-2-ene 2-methylbut-1-ene only .......................................... Q14: The enthalpy changes (ΔH values) for two reactions are given below. S(s) + O2 (g) → SO2 (g) ΔH = -297 kJ SO2 (g) + 1 /2 O2 (g) → SO3 (g) ΔH = -101 kJ What is ΔH for the following reaction? S(s) + 3 /2 O2 (g) → SO3 (g) A) B) C) D) -398 kJ -196 kJ +196 kJ +398 kJ .......................................... © H ERIOT-WATT U NIVERSITY 205 206 TOPIC 7. END OF UNIT TEST Q15: For the reaction: PCl3 (g) + Cl2 (g) PCl5 (s) ΔH = -126 kJ Which of the following changes will cause the greatest increase in the percentage of PCl5 in the above equilibrium mixture? a) b) c) d) Decrease in temperature, decrease in pressure Decrease in temperature, increase in pressure Increase in temperature, decrease in pressure Increase in temperature, increase in pressure .......................................... Sulfur burns in oxygen according to the equation S(s) + O 2 (g) → SO2 (g) 0.642 g of sulfur is placed in a vessel containing 0.25 l of oxygen. Under these conditions the molar volume of oxygen is 25.0 l mol -1 . Q16: How many moles of sulfur are present? Give your answer to 2 decimal places. .......................................... Q17: By calculating the number of moles of oxygen, which element is in excess? .......................................... Graph A shows the volume of hydrogen gas produced against time when an excess of magnesium is added to 50 cm 3 of hydrochloric acid of concentration 1 mol l-1 at 20◦ C. Graph B was obtained when the reaction was repeated with excess magnesium and hydrochloric acid of the same concentration but at a different temperature. Q18: Which reaction was at the higher temperature? a) A b) B © H ERIOT-WATT U NIVERSITY TOPIC 7. END OF UNIT TEST .......................................... Q19: How many cm3 of acid was required to produce graph B? .......................................... The apparatus shown can be used to prepare iron(III) chloride. Q20: On the diagram, highlight a substance which contains metallic bonding. .......................................... Q21: By considering the method of production, predict the type of bonding in iron(III) chloride. .......................................... Q22: Which of the following describes the bonding in the chlorides across Period 3 from NaCl to SCl2 ? a) b) c) d) Ionic → polar covalent Ionic → polar covalent → pure covalent Polar covalent → ionic Ionic → pure covalent → polar covalent .......................................... © H ERIOT-WATT U NIVERSITY 207 208 TOPIC 7. END OF UNIT TEST The potential energy diagram below represents the decomposition of hydrogen iodide: 2HI(g) → H2 (g) + I2 (g) Q23: What is the value for the activation energy (E A ) for the above reaction? .......................................... Q24: What would be the enthalpy change (ΔH) for the reaction? H2 (g) + I2 (g) → 2HI(g) .......................................... Q25: The use of a catalyst would . . . . . . the activation energy in part 1. .......................................... Q26: The use of a catalyst would . . . . . . the enthalpy change in part 2. .......................................... Q27: The enthalpy change for the reaction 1 / H (g) + 1 / I (s) → HI(g) 2 2 2 2 is known as the enthalpy of formation of hydrogen iodide. It differs from the equation above in two respects: 1. it involves the formation of one mole of HI, 2. and the reactants are in their standard states, so iodine is a solid. Given that the enthalpy of sublimation of iodine I2 (s) → I2 (g) is ΔH = + 62 kJ calculate the enthalpy of formation of hydrogen iodide. .......................................... Q28: If the enthalpy of formation of hydrogen chloride is -92 kJ mol -1 , predict a value for the enthalpy of formation of hydrogen bromide. © H ERIOT-WATT U NIVERSITY TOPIC 7. END OF UNIT TEST .......................................... Q29: Identify a peptide link on the following diagram. .......................................... Q30: Identify the phenyl group on the following diagram. .......................................... Q31: On hydrolysis, this section of protein produces three amino acids, none of which can be made by the human body. What name is given to such amino acids? .......................................... © H ERIOT-WATT U NIVERSITY 209 210 TOPIC 7. END OF UNIT TEST Q32: Which of the following amino acids could NOT be produced on hydrolysis of this section of protein? a) b) c) d) .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 7. END OF UNIT TEST The following diagram shows some reactions of ethene and ethyne. Q33: Compounds A and C are isomers. Which is the structural formula of compound C? a) b) c) d) .......................................... Q34: Ethanol can be made industrially by the catalytic hydration of ethene as shown in the diagram. If the process is 85% efficient, how many kg of ethanol will be produced from 112 kg of ethene? Give your answer to one decimal place. .......................................... © H ERIOT-WATT U NIVERSITY 211 212 TOPIC 7. END OF UNIT TEST The enthalpy changes for three reactions are shown below. C(s) + O2 (g) → CO2 (g) ΔH1 H2 (g) + O2 (g) → H2 O(l) ΔH2 CH4 (g) + 2O2 (g) → CO2 (g) + 2H2 O(l) ΔH3 Q35: What is the ΔH value for the following reaction? C(s) + 2H2 (g) → CH4 (g) A) ΔH1 + 2ΔH2 - ΔH3 B) ΔH3 - 2ΔH2 - ΔH1 C) ΔH1 + ΔH2 + ΔH3 D) ΔH1 + ΔH2 - ΔH3 .......................................... Methanol is manufactured by the reaction of carbon monoxide and hydrogen in synthesis gas. CO(g) + 2H2 (g) CH3 OH(g) ΔH = - 91 kJ mol-1 Q36: What is the atom economy of this reaction? .......................................... Q37: In the manufacture of methanol, which way would the equilibrium move if the pressure was increased? .......................................... Q38: Would this increase or decrease the yield of methanol? .......................................... Q39: In the manufacture of methanol, what would be the effect on the yield of product of decreasing the temperature? .......................................... Q40: The process used to be operated at 300 atmospheres pressure, but improved catalysts allow an efficient process at 100 atmospheres. Give one reason why 100 atm. is better for industry than 300 atm. .......................................... Q41: What effect would the improved catalyst have on the position of equilibrium?. .......................................... Q42: HgCl2 (aq) + SnCl2 (aq) → Hg(l) + SnCl4 (aq) What ion is oxidised in the above redox reaction? a) Cl- (aq) b) Sn2+ (aq) © H ERIOT-WATT U NIVERSITY TOPIC 7. END OF UNIT TEST c) Sn4+ (aq) d) Hg2+ (aq) .......................................... Q43: Which of the following reactions can be classified as reduction? a) b) c) d) CH3 CH2 COCH3 → CH3 CH2 CH(OH)CH3 CH3 CH(OH)CH3 → CH3 COCH3 CH3 CH2 OH → CH3 COOH CH3 CH2 CHO → CH3 CH2 COOH .......................................... Q44: Which of the following is a redox reaction? a) b) c) d) ZnO + 2HCl → ZnCl 2 + H2 O ZnCO3 + 2HCl → ZnCl2 + H2 O + CO2 Zn(OH)2 + 2HCl → ZnCl2 + 2H2 O Zn + 2HCl → ZnCl 2 + H2 .......................................... Q45: In which of the following reactions is a positive ion reduced? a) b) c) d) Sulfate → sulfite Gold(II) → gold(III) Iron(III) → iron(II) Bromide → bromine .......................................... Q46: In which of the following reactions is the hydrogen ion acting as an oxidising agent? a) b) c) d) Fe + 2HCl → FeCl 2 + H2 KOH + HNO3 → KNO3 + H2 O MgCO3 + H2 SO4 → MgSO4 + H2 O + CO2 CH3 COONa + HCl → NaCl + CH 3 COOH .......................................... The water in swimming pools is often disinfected by adding "chlorinated lime". This produces chlorate(I) ions (OCl- ) in solution, which effectively kill pathogens, as long as their concentration is sufficiently high. Q47: One method of estimating the OCl- concentration is to take a known volume of pool water and add an excess of potassium iodide solution. The reactions below produce iodine which can then be measured. Fill in the gaps to complete the ion-electron equations below. .......................................... © H ERIOT-WATT U NIVERSITY 213 214 TOPIC 7. END OF UNIT TEST Q48: .......................................... Q49: Which ionic equation is correct for the whole reaction? a) b) c) d) OCl- + 2H+ + 2I- → Cl- + H2 O + I2 OCl- + H+ + 2I- → Cl- + H2 O + I2 OCl- + 2H+ + I- → Cl- + H2 O + I2 2OCl- + 2H+ + I- → 2Cl- + H2 O + I2 .......................................... Q50: If 100 cm3 of the pool water produced 0.002 moles of iodine, what was the concentration of OCl- in mol l-1 ? .......................................... Q51: In order to be effective the concentration of chlorate(I) should be between 0.4 and 1.5 mg l-1 . What is the concentration of OCl- in mg l-1 ? .......................................... Q52: Using the table of standard reduction potentials, which of these redox reactions will take place? a) Cl2 (g) + 2Br- (aq) → 2Cl- (aq) + Br2 (aq) b) Br2 (l) + 2Fe2+(aq) → 2Br- (aq) + 2Fe3+(aq) c) Ca2+ (aq) + Cu(s) → Ca(s) + Cu2+ (aq) d) Zn2+ (aq) + Cu(s) → Zn(s) + Cu2+ (aq) e) Mg2+ (aq) + H2 (g) → Mg(s) + 2H+ (aq) f) Cu(s) + Cu2+ (aq) → 2Cu+ (aq) .......................................... Q53: The iodate ion, IO 3 - , can be converted to iodine. Which is the correct ion-electron equation for the reaction? a) b) c) d) 2IO3 - (aq) + 12H+ (aq) + 11e- → I2 (aq) + 6H2 O(l) 2IO3 - (aq) + 12H+ (aq) → 2I- (aq) + 6H2 O(l) 2IO3 - (aq) + 12H+ (aq) → 2I- (aq) + 6H2 O(l) IO3 - (aq) + 6H+ (aq) → I- (aq) + H2 O(l) .......................................... Q54: A major source of iodine is Caliche, mined in Chile. The mass of iodine in a 10 g sample of caliche can be determined by dissolving the sample in water and adding hydrogen peroxide solution to oxidise the iodide to iodine molecules. The ion-electron equation for the reduction reaction is shown. H2 O2 (aq) + 2H+ + 2e- → 2H2 O(l) Write a balanced redox equation for the reaction of hydrogen peroxide with iodide ion. .......................................... © H ERIOT-WATT U NIVERSITY TOPIC 7. END OF UNIT TEST 215 Q55: Using starch solution as indicator, the iodine solution is then titrated with sodium thiosulfate solution to determine the mass of iodine in the sample. The balanced equation for the reaction is shown. 2Na2 S2 O3 (aq) + I2 (aq) → 2NaI(aq) + Na2 S4 O6 (aq) In an analysis of a sample, 13.25 cm 3 of 0.0100 mol l-1 sodium thiosulfate solution was required to reach the end-point. Calculate the mass of iodine present in the sample of caliche. .......................................... Q56: Hydrogen peroxide is used in gels to whiten teeth. The ion-electron equation for the oxidation of hydrogen peroxide is: H2 O2 → O2 + 2H+ + 2eUsing your knowledge of chemistry, comment on possible methods for measuring and comparing the concentration of hydrogen peroxide present in two different gels. (This question is worth 3 marks) .......................................... Q57: Concentrated solutions of hydrogen peroxide are used in the propulsion systems of torpedoes. Hydrogen peroxide decomposes naturally to form water and oxygen: 2H2 O2 (aq) → 2H2 O() + O2 (g) ΔH = -196.4 kJ mol-1 Transition metal oxides act as catalysts in the decomposition of the hydrogen peroxide. Unfortunately, there are hazards associated with the use of hydrogen peroxide as a fuel in torpedoes. It is possible that a leak of hydrogen peroxide solution from a rusty torpedo may trigger an explosion. Using your knowledge of chemistry, comment on why this could happen. (This question is worth 3 marks) .......................................... .......................................... © H ERIOT-WATT U NIVERSITY 216 GLOSSARY Glossary Avogadro's constant this is the number of atoms in one mole of an element. In particular, it is the number of atoms in 12.0g of the isotope carbon-12. This number is given the symbol L and has a value of 6.02 × 10 23 Bond enthalpies bond enthalpy is the amount of energy needed to break one mole of a bond in a gaseous molecule Chromatography an analytical method where mixtures are separated into their components by partitioning between a stationary and mobile phase. The stationary/mobile phases are solid/liquid in paper and thin layer chromatography, and liquid/gas in gas-liquid chromatography. Dynamic equilibrium a dynamic equilibrium is achieved when the rates of two opposing processes become equal, so that no net change results Endothermic reactions absorb heat energy from the surroundings End-point the point at which the reaction is just complete Enthalpy change the name given to the amount of heat evolved or absorbed in a reaction carried out at constant pressure. It is given the symbol ΔH, read as "delta H" Enthalpy of combustion is the enthalpy change that occurs when 1 mole of a substance is burned completely in oxygen Enthalpy of neutralisation is the energy change (in kJ) when an acid is neutralised to form 1 mole of water Enthalpy of solution is the energy change (in kJ) when 1 mole of the substance dissolves in water Equilibrium chemical equilibrium is the state reached by a reaction mixture when the rates of forward and reverse reactions have become equal Exothermic reactions release heat energy, which is given up to the surroundings © H ERIOT-WATT U NIVERSITY GLOSSARY Feedstocks a feedstock is a reactant from which other chemicals can be extracted or synthesised. Feedstocks are themselves derived from raw materials either by physical separation or by chemical reaction Formula unit the term 'formula unit' is a general term. A formula unit may be an atom (for all elements which do not exist as diatomic molecules), a molecule (for all covalent molecular substances) or the simplest ratio of atoms or ions (for network or lattice substances). Hess's law the enthalpy change for a chemical reaction is independent of the route taken, providing the starting point and finishing point is the same for both routes Ion-electron equations an ion-electron equation is a half-equation, either an oxidation or a reduction, which in combination of the opposite type, can be part of a complete redox equation Molar volume the molar volume is the volume occupied by one mole of a substance. For gases, the units used are mol-1 . (Note that some texts will quote the molar volume in units of decimetres cubed per mole (dm 3 mol-1 ) Oxidation an oxidation is a loss of electrons by a reactant in any reaction Oxidising agent an oxidising agent is a substance which accepts electrons Potential energy diagram shows the enthalpy of reactants and products, and the enthalpy change during a chemical reaction Reducing agent a reducing agent is a substance which donates electrons Reduction a reduction is a gain of electrons by a reactant in any reaction Retention time the time taken for an individual peak to traverse the gas-liquid chromatographic column after the injection time Specific heat capacity relates the energy change in a liquid to the change in temperature. For water it has a value of 4.18 kJ kg-1 ◦ C-1 . In other words, when 1 kg of water absorbs 4.18 kJ of heat its temperature will rise by 1◦ C. © H ERIOT-WATT U NIVERSITY 217 218 GLOSSARY Standard solution a solution of accurately known concentration Theoretical yield the theoretical yield is the maximum possible amount of product in a reaction, i.e. all of the reactant(s) have been converted into product Thermochemical equation states the enthalpy change for the reaction defined, with reactants and products in the states shown Titration determines the volume of reactant solution required to react completely with the test solution Volumetric analysis involves analysis using a solution of accurately known concentration in a quantitative reaction to determine the concentration of another substance © H ERIOT-WATT U NIVERSITY HINTS Hints for activities Topic 1: Getting the most from reactants Test your prior knowledge Hint 1: 1. Write a balanced equation. 2. Identify mole ratio. 3. Calculate number of moles of potassium hydroxide (n = cv). 4. Use mole ratio to determine number of moles of nitric acid. 5. Calculate concentration of nitric acid (c = n/v). Topic 3: Chemical energy Test your prior knowledge Hint 1: Question 4 • Identify equation to use the equation Eh = cmΔT. • Convert mass of water to kg. • Calculate ΔT. (Final Temperature - Initial Temperature). • Use equation to solve. © H ERIOT-WATT U NIVERSITY 219 220 ANSWERS: TOPIC 1 Answers to questions and activities 1 Getting the most from reactants Test your prior knowledge (page 4) Q1: d) All of the above Q2: d) 14.5 Q3: b) 0.2 mol l-1 Sulfuric acid manufacture (page 6) Q4: a) Sulfur, Air and Water. Q5: a) Readily available and c) Available at a suitable cost. Clearly air and water are very abundant and free. (Why do you think that major sulfuric acid plants are built near rivers?) Sulfur is available as a mineral deposit, but it is not sustainable, although it is not thought to be in short supply. Q6: a) To make the reaction more economical. A single catalyst bed converts only part of the sulfur dioxide to sulfur trioxide. This is because the reaction releases heat which inhibits further reaction. So the mixture is cooled and passed through further catalysts, so that a large proportion of the sulfur dioxide is oxidised to trioxide. This makes the process more economic. Q7: a) Yes There are no products other than H 2 SO4 . However, the catalyst will eventually become ineffective and have to be disposed of. Q8: The burning of sulfur and conversion in the catalyst beds generate heat which can be used to heat up other processes that require heat, or to heat business premises. Answers from page 11. Q9: b) 16.05 Q10: c) 4FeS2 + 11O2 → 2Fe2 O3 + 8SO2 Q11: b) 217.9 Q12: d) 61.16 Q13: 23.53 g CH 3 OH; 89.71 g C6 H5 COOH 1. Write the formulae for methanol, benzoic acid and methyl benzoate then calculate the relative formula masses. (CH3 OH - 32; C6 H5 COOH - 122; C6 H5 COOCH3 - 136) 2. Write a balanced equation. (CH3 OH + C6 H5 COOH → C6 H5 COOCH3 + H2 O) © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 1 221 3. From the balanced equation, write down the mole ratios, then the ratio of masses. (1 mol CH3 OH reacts with 1 mol C6 H5 COOH to make 1 mol C 6 H5 COOCH3 32g CH3 OH reacts with 122 g C6 H5 COOH to make 136 g C 6 H5 COOCH3) 4. Use mathematical ratios to calculate the required quantities of alcohol and acid to make 100 g ester. Answers from page 15. Q14: We know the total mass of the nails and we know the mass of one nail. total mass mass of one 10.75 = 0.43 = 25 The number of nails = Nuts and bolts (page 15) Q15: The number of nuts = = However, 1 tonne = So, the number of nuts = = total mass mass of one 3 tonnes 3g 1000 kg = 1000000 g 3000000 3 1000000 (one million) Q16: 1000000 Q17: 2 Avogadro's constant calculations (page 16) Q18: total mass of helium mass of one He atom 4.0 g = 4.0 amu 1.0 g = 1.0 amu Number of atoms (L) = Q19: L Q20: 27.0 Q21: 63600 © H ERIOT-WATT U NIVERSITY 222 ANSWERS: TOPIC 1 Formula units (page 17) Q22: atom Q23: molecule Q24: c) one sodium ion and one chloride ion. Q25: c) one silicon atom and two oxygen atoms. Calculations using Avogadro's constant (optional) (page 20) Q26: The GFM for carbon dioxide is 44.0 g. Given Required Mass of CO2 Number of molecules Key relationship 1 mole contains L formula units For CO2 44 g contains 6.02 × 1023 molecules In this case 0.22 g contains 0.22/44 × 6.02 × 10 23 molecules Answer 0.22 g CO2 contains 3.01 × 1021 molecules Here is the calculation: 44.0 g contains 6.02 × 10 23 molecules 1.0 g contains 6.02 × 1023 44.0 molecules 0.22 × 6.02 × 1023 44.0 10 23 molecules 0.22 g contains = 0.0301 × molecules = 3.01 × 1021 molecules Q27: Direction Key relationship Given → Required number of particles → mass 6.02 × 1023 formula units are in one GFM The GFM for calcium carbonate is 100.0 g. 6.02 × 1023 formula units in 1 GFM © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 1 223 6.02 × 1023 formula units in 100.0 g 1 formula unit in 100.0 g 6.02 × 1023 3.612 × 1020 formula units in = 3.612 6.02 3.612 × 1020 × 100.0 6.02 × 1023 g × 10-1 g = 0.6 × 10-1 g Q28: a) Direction Key relationship Given → Required mass → number of molecules one GFM contains 6.02 × 10 23 molecules The GFM for methane is 16.0 g. 16.0 g contains 6.02 × 10 23 molecules 6.02 × 1023 molecules 16.0 6.02 × 1023 molecules 0.112 g contains 0.112 × 16.0 23 = 0.04214 × 10 molecules 1.0 g contains = 4.214 × 1021 molecules b) Methane has the formula CH 4 and so there are five atoms in each molecule. The number of atoms = 5 × 4.214 × 10 21 = 2.107 × 1022 Q29: Magnesium nitrate has the formula Mg(NO 3 )2 . Each formula unit contains three ions (one Mg2+ and two NO3 - ). The GFM of magnesium nitrate is 148.3 g. 148.3g contains 6.02 × 10 23 formula units 6.02 × 1023 formula units 148.3 37000 × 6.02 × 1023 formula 37000g contains 148.3 = 1502 × 1023 formula units 1.0g contains = 1.502 × 1026 formula units Number of ions = 3 × 1.502 × 10 26 = 4.506 × 1026 ions © H ERIOT-WATT U NIVERSITY units 224 ANSWERS: TOPIC 1 Q30: Potassium sulfate has the formula K 2 SO4 and a GFM of 174.3 g. One formula unit contains three ions (two K+ and one SO 4 2- ) 9.03 × 1021 ions = 3.01 × 1021 formula units 6.02 × 1023 formula units in GFM 6.02 × 1023 formula units in 174.3 g 1 formula unit in 174.3 6.02 × 1023 g 3.01 × 1021 formula units in 3.01 × 1021 × 174.3 6.02 × 1023 g = 87.15 × 10-2 g = 0.8715 g Molar volume (page 24) Q31: Initial mass of container and gas = 124.86 g Final mass of container and gas = 124.77 g Mass of hydrogen = 0.09 g GFM of hydrogen = 2.0 g Direction of calculation is mass ⇒ volume 0.09 g occupy 1 l 1.0 g occupy 2.0 g occupy 1 0.09 2.0 0.09 l l = 22.2 Molar volume of hydrogen is 22.2 l mol -1 Q32: Initial mass of container and gas = 124.86 g Final mass of container and gas = 122.88 g Mass of carbon dioxide = 1.98 g GMF of carbon dioxide = 44.0 g Direction of calculation is mass ⇒ volume 1.98 g occupy 1 l 1 1.98 l occupy 44.0 1.98 1.0 g occupy 44.0 g l = 22.2 Molar volume of carbon dioxide is 22.2 mol -1 Q33: They are all the same © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 1 Answers from page 26. Q34: 22.86 Q35: 22.65 Q36: 22.19 Answers from page 26. Q37: 30.0 Molar volume - Further practice (page 27) Q38: 16 l mol-1 Q39: 0.0321 moles Q40: 16500cm 3 Combustion of methane - Further practice (page 31) Q41: Start by writing the balanced equation, mole relationship and volume relationship. Work out which reactant is in excess: 1 volume of propane requires 5 volumes of oxygen 200 cm3 of propane require exactly 1000 cm 3 of oxygen There are 1300 cm3 of oxygen and so oxygen is in excess. © H ERIOT-WATT U NIVERSITY 225 226 ANSWERS: TOPIC 1 1000 cm3 of the oxygen is used up. So 300 cm 3 remains unreacted. 600 cm3 of carbon dioxide is formed. So the total volume of the resulting gas mixture is 900 cm3 . Q42: Start by writing the balanced equation, mole relationship and volume relationship. Work out which reactant is in excess: 1 volume of ethene requires 3 volumes of oxygen 400 cm3 of ethene require exactly 1200 cm3 of oxygen There are only 900 cm3 of oxygen and so ethene is in excess. 300 cm3 of the ethene is used up. So 100 cm 3 of ethene remains unreacted. 600 cm3 of carbon dioxide is formed. So the total volume of the resulting gas mixture is 700 cm3 . © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 1 227 Answers from page 35. Q43: a) 64 g ⇒ 26 g 26 g 1g ⇒ 64 26 × 2.56 g 2.56 g ⇒ 64 = 1.04 g Theoretical yield is 1.04 g. b) actual × 100 theoretical 0.99 × 100 = 1.04 = 95.2% Percentage yield = Q44: a) 26 g ⇒ 168 g 168 g 1g ⇒ 26 168 × 1.3 g 1.3 g ⇒ 26 = 8.4 g © H ERIOT-WATT U NIVERSITY 228 ANSWERS: TOPIC 1 Theoretical yield is 8.4 g. b) actual × 100 theoretical 7.14 × 100 = 8.4 = 85% Percentage yield = Calculations involving percentage yields - Further practice (page 36) Q45: 56.3 tonnes Q46: 90.6% Q47: 12.43 grams Q48: 8.08 grams Answers from page 37. Q49: Using relative atomic masses from p. 1 of the data booklet, mass of CaO is 56, mass of CaCO3 is 100. substituting into the equation: atom economy = (56/100) × 100 = 56% The atom economy of the production of calcium oxide from calcium carbonate is 56% Q50: Using relative atomic masses from p. 1 of the data booklet, mass of SO 3 is 80.1, mass of SO2 is 64.1, mass of 1 /2 O2 is 16. substituting into the equation: atom economy = (80.1/80.1) × 100 = 100% The atom economy of the production of sulfur trioxide from sulfur dioxide and oxygen is 100% Q51: All the materials go to produce the product. There is no waste from the process. Q52: Rearrangements have an identical mass of reactant and product, so have 100% atom economy. © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 1 229 Ibuprofen (page 38) Q53: Used in ibuprofen Reagent Not used Formula Mr Formula Mr Formula Mr C10 H14 134 C10 H13 133 H 1 C 4 H 6 O3 102 C2 H3 O 43 C 2 H 3 O2 59 H2 2 H2 2 0 CO 28 CO 28 0 Ibuprofen Total C15 H22 O4 266 C13 H18 O2 Waste 206 C 2 H 4 O2 60 Q54: atom economy = (206/266)100 = 77% Q55: Q56: Step 1 - 90% yield = 0.9 (so for each 100 g of product you should theoretically get, there is only 0.9 i.e. 90 g produced); Step 2 - 0.9 × 0.9 = 0.81 = 81% (should get 90 g but only 0.9 of this, so 81 g) ; Step 3 - 0.9 × 0.9 × 0.9 = 0.73 = 73% ... Step 6 - (0.9)6 = 0.53 = 53% Q57: 73% Q58: atom economy = mass of product / mass of starting materials percentage atom economy = (206/514.5)100 = 40% © H ERIOT-WATT U NIVERSITY 230 ANSWERS: TOPIC 1 Q59: Converting to grams, 3000 tonnes is 3 × 10 9 g Number of tablets taken is 3 × 10 9 /0.2 = 1.5 × 1010 Q60: 250 Q61: Addition reactions Q62: The two new stages are addition reactions which have a 100% atom economy. These replace the wasteful previous five stages. Answers from page 43. Q63: Each sandwich needs two slices of bread and one slice of cheese, so you can make 12 sandwiches. This will use all 12 cheese slices and 24 slices of bread. What remains? 4 slices of bread. The bread can be said to be 'in excess'. The cheese is 'limiting', since the number of cheese slices limits the number of sandwiches you can prepare. Calculating excess - Tutorial examples (page 45) Q64: Solution • Write a balanced equation, including the mole ratios • Choosing aluminium and calculating quantities involved © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 1 • Calculate quantities of the other reactant involved • Use the balanced equation to work out which reactant is in excess In this case the aluminium to iron (III) oxide react in a 2:1 ratio. So 1.85 mol of aluminium would require 0.925 mol iron (III) oxide. (There is not enough iron (III) oxide, aluminium is in excess.) So 0.63 mol iron (III) oxide would require 1.26 mol of aluminium. Excess aluminium = 1.85 - 1.26 = 0.59 mol • Calculate excess present The aluminium is in excess by 0.59 mol. 1 mol aluminium = 27 g So 0.59 mol aluminium = 0.59 × 27 g 0.59 mol aluminium = 15.93 g Excess aluminium = 15.93 g Q65: hydrochloric acid Q66: 0.3 Q67: hydrochloric acid Q68: 0.02 Q69: 0.06 Q70: copper (II) oxide Q71: copper (II) oxide Q72: 0.70 Q73: 2.0 © H ERIOT-WATT U NIVERSITY 231 232 ANSWERS: TOPIC 1 End of topic 1 test (page 48) Q74: 228.6 cm3 mol-1 Q75: B and C. 32g CH 4 and 4 g H2 Q76: C. CH4(g) + 2O2 (g) → CO2 (g) + 2H2 O(l) Q77: 100 cm3 Q78: 100 cm3 Q79: 600 cm3 Q80: C. 4 Q81: A. 1.0 litre Q82: 85% Q83: 64% Q84: b) feedstocks. Q85: A: The mass of marble chips left over after the reaction had finished. Q86: B: 80% Q87: b) heat exchangers and c) improved catalysts Q88: Q89: 16.2 tonnes © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 1 Q90: 38% Q91: 2CH3 CH(OH)CH3 + O2 → 2CH3 COCH3 + 2H2 O Q92: 76% Q93: Any three from: • percentage yield; • availability of raw materials; • potential sales of co-product (phenol); • energy efficiency of the process; • costs of building and running the plant. © H ERIOT-WATT U NIVERSITY 233 234 ANSWERS: TOPIC 2 2 Chemical equilibria Test your prior knowledge (page 55) Q1: a) Equilibrium is a situation where forward and reverse reactions take place at the same rate. Q2: b) A reaction which proceeds in both directions at the same time. Q3: d) H2 0(l) H+ (aq) + OH- (aq) Establishing dynamic equilibrium (page 60) Q4: 6 Q5: 4 Q6: 2 Q7: b) remain constant, but not necessarily equal. Q8: 2 Q9: c) There is the same equilibrium mixture as before. Q10: This is an equilibrium because the rate of forward reaction (left to right) is equal to the rate of backward reaction (right to left). It is dynamic because both reactions continue and although it looks, to an external observer, as if no change is occurring, products continue to be formed and undergo further reaction to reform reactants. (This answer could gain 2 marks.) Answers from page 62. Q11: 4 Q12: 6 Q13: endothermic Q14: This is to be expected as Le Chatelier's principle would predict that an increase in temperature will favour the reaction which absorbs heat energy, the endothermic reaction, which is the forward reaction. This is shown in the colour of the gas, which is dark brown. This answer could gain 2 marks. Q15: 2 Q16: exothermic Q17: This is to be expected as Le Chatelier's principle would predict that a decrease in temperature will favour the reaction which gives out heat energy, the exothermic reaction, which is the reverse reaction. This is shown in the colour of the gas, which is very pale, almost colourless. This answer could gain 2 marks. © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 2 Equilibrium and pressure (page 64) Q18: decreased Q19: decreased Q20: left Q21: Le Chatelier's principle states that if the conditions of a chemical system at equilibrium are changed, the system responds by minimising the effect of the changes. Increasing the pressure moves the equilibrium to the side of the equation with fewer gas molecules as this reduces the pressure. The position of equilibrium shifts to the side with the N2 O4(g) (the left). The colour lightens. A full answer like this would be worth two or three marks in an examination situation. Q22: Le Chatelier's principle states that if the conditions of a chemical system at equilibrium are changed, the system responds by minimising the effect of the changes. Decreasing the pressure shifts the equilibrium to the side of the equation with a larger volume and more gas molecules as this increases the pressure. The system shifts to the side with the NO2 (g) (the right). This is shown in the observation that the colour darkens due to the increase in the number of NO 2 (g) molecules. Changing concentration (page 65) Q23: nitrogen Q24: increased Q25: right Q26: Le Chatelier's principle states that if the conditions of a chemical system at equilibrium are changed, the system responds by minimising the effect of the changes. Increasing the concentration of nitrogen shifts the position of equilibrium to the side of the equation which uses up the nitrogen, thus producing more ammonia. The equilibrium position shifts to the right. Q27: Le Chatelier's principle suggests that increasing the concentration of nitrogen shifts the position of equilibrium to the side of the equation which uses up the nitrogen. As this happens, hydrogen has to be used up to react with the nitrogen and form ammonia, thus the hydrogen line drops after nitrogen is added. Answers from page 68. Q28: decreased Q29: Le Chatelier's principle states that if the conditions of a chemical system at equilibrium are changed, the system responds by minimising the effect of the changes. Decreasing the concentration of ammonia causes an initial dip in the concentration, but the system then responds. The equilibrium shifts to the side of the equation which minimises the change, by producing more ammonia. The equilibrium shifts right. © H ERIOT-WATT U NIVERSITY 235 236 ANSWERS: TOPIC 2 Answers from page 69. Q30: low Q31: Is the forward reaction exothermic or endothermic? According to Le Chatelier's principle will high or low temperature favour the forward reaction? (Remember: Le Chatelier's principle states that if the conditions of a chemical system at equilibrium are changed, the system responds by minimising the effect of the changes). Since the forward reaction is exothermic, low temperature favours the forward reaction. Q32: a) Less than 10% ammonia Q33: At low temperatures the reaction rate is very slow and it takes a long time to reach equilibrium. At 400 ◦ C the reaction reaches equilibrium faster and although the yield is lower, separating off the ammonia by cooling and condensing, and recycling the unreacted gases gives a continuous stream of ammonia at a reasonable rate. Answers from page 70. Q34: high Q35: Is there an increase or decrease in volume in the forward reaction? According to Le Chatelier's principle will high or low pressure favour the forward reaction? (Remember: Le Chatelier's principle states that if the conditions of a chemical system at equilibrium are changed, the system responds by minimising the effect of the changes). The forward reaction occurs with a decrease in volume. High pressure favours this decrease in volume. Q36: High pressure processes are very expensive to maintain, have high construction and energy costs and are less safe. At 200 atmospheres there is still a reasonable yield of ammonia, the pressure is high enough to force the equilibrium to the ammonia side and the separating of ammonia and recycling of unreacted gases ensures an economic process. Summary of the Haber process (page 72) Q37: © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 2 End of topic 2 test (page 74) Q38: B. rate of forward reaction equals the rate of reverse reaction. Q39: C. remain constant. Q40: B. is a reversible reaction. Q41: A. Low temperature, high pressure Q42: C. the proportion of sulfur trioxide to decrease. Q43: D. both reactant gases to increase concentration. Q44: A. no change in the position of equilibrium. Q45: A. KCl(s) Q46: B and D: KOH(s) and AgNO 3 (s) Q47: A and C: It decreases the time required for equilibrium to be established. and It lowers the activation energy of the reverse reaction. Q48: endothermic Q49: The concentration of nitrogen dioxide would increase. Q50: 450◦ C Q51: They are recycled. © H ERIOT-WATT U NIVERSITY 237 238 ANSWERS: TOPIC 3 3 Chemical energy Test your prior knowledge (page 83) Q1: a) Combustion Q2: c) Li2 CO3 (s) + 2HCl(aq) → 2LiCl(aq) + CO2 (g) + H2 O(l) Q3: a) Exothermic Q4: b) 33.44 Q5: b) addition. Answers from page 88. Q6: c) It will decrease. Q7: c) Positive Answers from page 89. Q8: d) Endothermic Q9: a) Positive. Q10: Answers from page 93. Q11: d) ΔH = +283 kJ mol-1 Q12: b) ΔH = Hproducts - Hreactants Q13: c) Exothermic Q14: d) ΔH = -1411 kJ mol-1 © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 3 Q15: -6835 kJ Q16: +150 kJ mol-1 Q17: 4NH3 (g) + 5O2 (g) → 4NO(g) + 6H2 O(g) Q18: -906 kJ Q19: -226.5 kJ mol-1 Q20: 1359 kJ Determination of the enthalpy of combustion of an alcohol (page 97) Q21: Some of the heat from the burning will have been lost completely from the equipment, e.g. in heating the air. You took account only of the heat given to the water, but the container and thermometer would also take some heat to warm up. Perhaps the alcohol did not burn completely to water and CO 2 . All these effects mean that the temperature rise noted was lower than it should have been, resulting in a smaller value for ΔH c . Enthalpy of combustion questions (page 99) Q22: b) Butane Q23: 68.9 ◦ C Q24: -522.5 kJ mol-1 An experiment to determine the enthalpy of solution of ammonium nitrate (page 100) Q25: You need the mass of water being heated (m), and the initial temperature. The value of c is a constant, obtained from the data booklet. Q26: The reaction occurring cools the solution. Once it is below the ambient temperature it will slowly absorb heat from the surroundings and rise in temperature. Q27: -1.6 ◦ C Answers from page 102. Q28: -40.1 kJ mol-1 Q29: 369 kJ Answers from page 104. © H ERIOT-WATT U NIVERSITY 239 240 ANSWERS: TOPIC 3 Q30: 0.1 mol of water Answers from page 105. Q31: H2 SO4 (aq) + 2KOH(aq) → K2 SO4 (aq) + 2H2 O(l) Q32: 21.4 ◦ C Q33: +13.6 ◦ C Q34: 0.10 kg Q35: 5.685 kJ Q36: 0.1 Q37: -56.85 kJ mol-1 Q38: They are almost the same Q39: Sulfuric acid and potassium hydroxide are almost completely ionised in solution, so if you write ionic equations for these two neutralisation reactions then remove spectator ions, they both reduce to the same equation. The same reactions must have the same ΔH value. which are both Answers from page 106. Q40: B Q41: c) +570 Answers from page 107. Q42: 4 Q43: They are activation energies for the reaction. Energy 2 is the large activation energy for the uncatalysed reaction; energy 1 shows the reduced activation energy in the presence of a catalyst. © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 3 241 Answers from page 110. Q44: Q45: E = c × m × ΔT = 4.18 × 0.025 × 18.1 = 1.89 kJ This is the energy released when 0.80 g of sodium hydroxide reacts. 1 mole of NaOH = 40 g 0.80 g releases 1.89 kJ 1.89 kJ 1.0 g releases 0.80 1.89 kJ 40.0 g releases 40 × 0.80 The enthalpy change = −94.5 kJ mol −1 (Note the negative sign since energy is released.) Answers from page 110. Q46: -41.8 kJ mol-1 Q47: 8.0 o C Q48: 0.025 kg Q49: 0.836 kJ Q50: 1 mole of NaOH = 40 g 0.80 g releases 0.836 kJ 0.836 kJ 1.0 g releases 0.80 0.836 kJ 40.0 g releases 40.0 × 0.80 = 41.8 kJ Q51: -41.8 kJ mol-1 Answers from page 111. Q52: -52.25 kJ mol-1 © H ERIOT-WATT U NIVERSITY 242 ANSWERS: TOPIC 3 Q53: 5.0 ◦ C Q54: 0.05 kg Q55: 1.045 kJ Q56: The solution from step 1 contains 0.80 g of NaOH 1 mole of NaOH = 40 g 0.80 g releases 1.045 kJ 1.045 kJ 1.0 g releases 0.80 0.836 kJ 40.0 g releases 40.0 × 0.80 = 52.25 kJ Q57: -52.25 kJ mol-1 Q58: -94.05 kJ mol-1 Application of Hess's law by enthalpy cycle diagram (page 112) Q59: -55.5 kJ Q60: +222 kJ Application of Hess's law by algebraic calculation (page 114) Q61: +55.5 kJ Q62: +333 kJ Bond enthalpies and the synthesis of ammonia (page 117) Q63: Bonds broken N≡N 3×H-H Total enthalpy change to break bonds ΔH 945 Bonds formed ΔH 6×N-H 6 × 388 Total enthalpy change on making bonds -2328 kJ 3 × 436 +2253 kJ ΔH to break bonds + ΔH to make bonds: 2253 + (-2328) = -75 kJ (You may have noticed earlier in the topic that the reaction enthalpy change was described as the enthalpy in the product bonds minus the enthalpy in the reactant bonds. © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 3 The equation above is for enthalpy changes and, therefore, includes an appropriate sign for exo- or endothermic changes. The overall change is then the sum of the changes.) The hydrogenation of ethene (page 118) Q64: d) 1 x C=C, 4 x C - H and 1 x H - H Q65: 1 × C - C and 6 × C - H Q66: Enthalpy change to break bonds = 612 + (4 × 412) + 436 = +2696 Enthalpy change to form bonds = -348 + (6 × -412) = -2820 Enthalpy change for reaction is +2696 + (-2820) = -124 kJ Q67: In this case average bond enthalpies were used, and since bond enthalpies are slightly different in different environments, the results will differ slightly. End of topic 3 test (page 121) Q68: A: ΔH = H(products) - H(reactants) Q69: A: -300 Q70: B: exothermic Q71: B: C2 H6 (g) + 31 /2 O2 (g) → 2CO2 (g) + 3H2 O(l) Q72: A: - 2680 kJ mol-1 Q73: B: bonds within the molecules. Q74: a) - 361 kJ mol-1 Q75: As molecules are getting larger, there are greater London Dispersion Forces between them which require more energy to be overcome." Q76: - 2688 kJ mol-1 Q77: B and E: 3O 2 (g) → 2O3 (g) ; ΔH = +142.7 kJ mol-1 and NH4 Cl (s) → NH4 + (aq) + Cl- (aq) ; ΔH = +15.0 kJ mol-1 Q78: C and E: NaOH(s) → Na + (aq) + OH- (aq) ; ΔH = -42.7 kJ mol-1 and NH4 Cl (s) → NH4 + (aq) + Cl- (aq) ; ΔH = +15.0 kJ mol-1 Q79: D: C3 H8 (g) + 5O2 (g) → 3CO2 (g) + 4H2 O(g) ; ΔH = -2220 kJ mol-1 Q80: C4 H10 (g) + 61 /2 O2 (g) → 4CO2 (g) + 5H2 O(g) © H ERIOT-WATT U NIVERSITY 243 244 ANSWERS: TOPIC 3 Q81: 1. The mass of water in the pot. 2. The temperature of the water at the start of the experiment. 3. The temperature of the water at the end of the experiment. Q82: - 1500 kJ mol-1 Q83: C: the same. Q84: - 28.3 kJ Q85: + 566 kJ Q86: a) ΔH3 = ΔH1 - ΔH2 Q87: 400 kJ Q88: - 156 kJ Q89: + 39 kJ Q90: - 848 kJ Q91: - 1605 kJ Q92: -788 kJ Q93: -858 kJ Q94: 3 moles Q95: +1367 kJ Q96: -279 kJ Q97: a) N(g) + 3H(g) → NH3 (g) Q98: a) H-H Q99: b) -115 Q100: Worked Solution Bond breaking Bond making 4 mol C-H = 4 × 412 = 1648 1 mol Br-Br = 194 3 mol C-H = 3 × 412 = 1236 1 mol C-Br = 276 1mol H-Br = 366 Total energy given out = -1878 kJ Total energy put in = +1842 kJ © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 3 245 ΔH = 1842 - 1878 = -36 kJ mol-1 Q101: Worked Solution Bond breaking Bond making 4 mol C-H = 4 × 412 = 1648 2 mol O=O = 2 × 498 = 996 Total energy put in = +2644 kJ 2 mol C=O = 2 × 743 = 1486 4 mol H-O = 4 × 463 = 1852 Total energy given out = -3338 kJ ΔH = 2644 - 3338 = -694 kJ mol-1 Q102: Worked Solution Bond breaking Bond making 1 mol C=C = 612 4 mol C-H = 4 × 412 = 1648 1 mol H-Br = 366 Total energy put in = +2626 kJ 1 mol C-C = 348 5 mol C-H = 5 × 412 = 2060 1 mol C-Br = 276 Total energy given out = -2684 kJ ΔH = 2626 - 2684 = -58 kJ mol-1 © H ERIOT-WATT U NIVERSITY 246 ANSWERS: TOPIC 4 4 Oxidising or reducing agents Test your prior knowledge (page 132) Q1: a) gain of electrons. Q2: c) Pb2+ (aq) + 2NO3 - (aq) + 2K+ (aq) + 2l- (aq) → Pb2+ (l- )2 (s) + 2K+ (aq) + 2NO3 - (aq) Q3: e) 5H2 O2 (l) + 2MnO4 - (aq) + 6H+ (aq) → 2Mn2+(aq) + 8H2 O(l) + 5O2 (g) Answers from page 136. Q4: oxidation Q5: c) Bromine Q6: iodide Q7: a) Top of the table. Q8: sulfur Q9: magnesium Q10: sulfur Q11: b) Br2 (1) Q12: a) Na (s) Q13: c) Sn2+ (aq) Displacement reactions (page 138) Q14: sulfate Q15: Zn → Zn2+ +2eQ16: Cu2+ +2e- → Cu Q17: d) Copper ions Q18: zinc atoms © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 4 Answers from page 139. Q19: These salts are soluble in water so can be used in reactions in solution - the commonest ones in chemistry. Q20: 2KMnO4 + 16HCl → 2KCl + 2MnCl 2 + 8H2 O + 5Cl2 Q21: The permanganate ion (MnO 4 - ) is the oxidising agent and the reducing agents are chloride ion (Cl- ) and hydrogen ion (H + ). Q22: MnO4 - is reduced to manganese(II) ion (Mn 2+ ). Chloride ion is oxidised to chlorine and hydrogen ion oxidised to water. Q23: K+ is a spectator ion. Answers from page 141. Q24: Looking at the reverse reaction it is much easier to see that glucose is oxidised by oxygen to carbon dioxide and water. In the case of photosynthesis, the carbon dioxide is reduced to glucose, and the water is oxidised to molecular oxygen. Q25: Increase in ppmv 390 - 315 = 75 ppmv CO 2 So volume of CO2 is: Total volume of atmosphere x ppmv x 10 -6 4 x 1020 x 75 x 10-6 = 3 x 1016 litres of CO2 Writing ion-electron equations (page 146) Q26: K → K+ + 1eQ27: Mg → Mg2+ + 2eQ28: Al → Al3+ + 3eQ29: F + 1e- → FQ30: N + 3e- → N3Q31: O + 2e- → O2- © H ERIOT-WATT U NIVERSITY 247 248 ANSWERS: TOPIC 4 Answers from page 147. Q32: b) At the bottom of the table. Q33: c) At the top of the table, in reverse. Q34: b) F2 (g) + 2e- → 2F- (aq) Q35: a) Na+ (aq) + e- → Na(s) Tutorial - simple ion-electron equations (page 149) Q36: Q37: Q38: Q39: © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 4 Answers from page 152. Q40: 1 Q41: 2 Q42: 2 Q43: a) Reactant Q44: b) Reduction Q45: The oxidation equation for Fe 2+ has to be multiplied by 2 and then added to the reduction equation: Tutorial - complex ion-electron equations (page 153) Q46: 3 Q47: 6 Q48: 2 Q49: b) Product Q50: a) Oxidation Q51: © H ERIOT-WATT U NIVERSITY 249 250 ANSWERS: TOPIC 4 Q52: Q53: Q54: The reduction ion-electron equation can be developed first: Combining this with the oxidation: © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 4 Estimation of vitamin C in fruit juices (page 156) Q55: orange juice Q56: 4.0 cm3 Q57: Number of moles of iodine used = 0.005 x 0.004 = 2.0 x 10 -5 mol Q58: a) 1:1 Q59: Number of moles of iodine used = Number of moles of vitamin C = 2.0 x 10 -5 mol This is present in 20.0 cm3 , therefore in one litre: Vitamin C concentration = 2.0 x 10 -5 mol divided by 0.02 litres (20.0 cm3 ) This is equal to 0.001 mol l -1 Q60: Vitamin C concentration = 0.001 mol l -1 In mg l -1 = 0.001 x 176 = 0.176 g l -1 = 176 mg l -1 . Q61: 340.9 cm3 Tutorial - calculations in redox titrations (page 158) Q62: 0.00225 mol l -1 Q63: Vitamin C concentration = 0.00225 mol l -1 In mg l -1 = 0.00225 x 176 = 0.396 g l -1 = 396 mg l -1 . Q64: 151.5 cm3 Q65: 0.009 moles Q66: 0.0045 mol l -1 Q67: 319.5 mg l -1 Q68: The working for the last three questions can be shown thus: © H ERIOT-WATT U NIVERSITY 251 252 ANSWERS: TOPIC 4 = Summary exercise (page 159) Q69: b) Fe2 O3 + 2Al → 2Fe + Al2 O3 Q70: c) Spectator Q71: c) Fe3+ + Al → Fe + Al3+ Q72: b) loss Q73: a) Al © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 4 Q74: d) Fe3+ Q75: a) Copper Q76: c) 3 kg End of topic 4 test (page 163) Q77: a) Cl2 + 2e- → 2ClQ78: b) accepts electrons. Q79: b) 2Br- → Br2 + 2eQ80: b) Fe2+ → Fe3+ + eQ81: b) Cu2+ → Cu + 2eQ82: c) Iodide ions will react with chlorine. Q83: d) Fe3+ Q84: c) Add 2 electrons to the left hand side. Q85: d) Iron(III) ions will react with tin(II) ions. Q86: Q87: Q88: d) Cl2 (g) Q89: c) Ca (s) Q90: e) Fe2+ (aq) Q91: 3 Q92: 6 Q93: 6 Q94: a) Reactant Q95: b) a reduction. Q96: 0.0016 Q97: a) 1:2.5 Q98: 0.16 Q99: 5.44 © H ERIOT-WATT U NIVERSITY 253 254 ANSWERS: TOPIC 5 5 Chromatography Test your prior knowledge (page 169) Q1: a) Distillation Q2: b) Filtration Q3: b) two solutions react to form a solid. Chromatography of blue and black inks (page 172) Q4: b) Black Q5: e) Green Q6: a) It is more polar than the red component. Q7: a) has the highest solubility in the solvent. Q8: c) Dark blue Q9: They are probably the same dyes in both cases since the R f values would be the same. If they were different materials, they would probably have moved different distances. Q10: If the sample components are hardly moving with the mobile phase, the polarity of the phase is too high, too similar to the stationary water. Try and use a less polar mobile phase, for example, octanol. © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 5 Chromatography of protein hydrolysates (page 175) Q11: 3 Q12: cysteine Q13: d) A, B and D Q14: Q15: Hydrolysed protein Y contains two amino acids (one mark). It contains cysteine because it has a spot which travelled the same as spot "C" (one mark). It contains an amino acid which cannot be named but it is not A, B or D (one mark). Q16: 3 Q17: phenylalanine Q18: alanine Q19: b) False Q20: Glycine with the lowest molecular mass travels furthest; but phenylalanine, with the highest molecular mass is not far behind, so mass does not determine the distance travelled. Q21: There are more than 20 amino acids found in proteins. Each one will move a different distance, so the developed chromatogram will have a large number of spots merging into a streak from the origin to the solvent front. © H ERIOT-WATT U NIVERSITY 255 256 ANSWERS: TOPIC 5 Q22: You need a very much longer distance for the amino acids to travel so that they can eventually separate. The problem with longer and longer plates is that i) as the spots travel diffusion causes them to spread out, and they also form a longer trail and ii) the solvent front slows down considerably as it travels further, so TLC is usually performed only on short plates. To separate many amino acids a technique called high pressure liquid chromatography (HPLC) is employed. The stationary phase is just like TLC (often silica), but the material is packed into a long column, so allowing time and distance for separating, and the mobile solvent is forced through the column with a high pressure pump. Variations of HPLC are very widely employed in a vast range of analytical systems from clinical chemistry to the petrochemical industry. Many of these are considerably automated. Gas-liquid chromatography (page 180) Q23: b) Pentane Q24: a) The retention time increases as mass increases. Q25: c) Xylene (C8 H10 ) Gel filtration (page 181) Q26: b) The larger molecules are excluded form the pores in the stationary beads. Q27: c) The sample molecules would elute from the column in a single peak. Q28: c) C, B then A Q29: There are two proteins, one has a molecular mass between 1,000 and 3,000 (probably about 2,000); the other has a molecular mass close to 6,000. Q30: No. There are only two molecular masses shown, but there could be more than one type of protein, with very similar molecular masses, under each peak. Q31: Note the increased area of the first (6,000) peak owing to the presence of standard and sample at that position. © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 5 Q32: You need a method that does not depend on size, but on some other property. You could try paper chromatography, which depends on polarity. If sample X produced more than two 'spots' there are more than two proteins. End of topic 6 test (page 184) Q33: Glycine and alanine Q34: There are three spots and so three amino acids. Q35: a) A and B Q36: c) Q37: b) A and D Q38: d) Branched alkanes have lower boiling points than n-alkanes with the same number of carbon atoms. Q39: a) Plant waxes Q40: d) Branched alkanes, such as pristane and phytane, are degraded more slowly than n-alkanes. Q41: They are oxidised (1 mark) to carbon dioxide and water (1 mark). Q42: The oxidation process required for total removal of an oil spill is mainly carried out by bacteria and fungi. These exist in the water, as does the oxygen. In order to allow these organisms to oxidise the hydrocarbons, they should be in as small a droplets as possible, with the largest area in contact with water. Detergents and emulsifiers, with the churning action of the sea, will break the oil into these small droplets. © H ERIOT-WATT U NIVERSITY 257 258 ANSWERS: TOPIC 6 6 Volumetric analysis Test your prior knowledge (page 191) Q1: a) Chromatography Q2: a) End point Q3: b) A salt and water Volumetric analysis equipment (page 192) Q4: a) A Q5: e) E Q6: d) D Q7: c) C Titration (page 193) Q8: 1. Write a balanced equation: HCl + KOH → KCl + H 2 O The mol relationship: 1 mol HCl reacts with 1 mol KOH 2. Moles of HCl used = concentration volume = 0.150 × 0.0345 = 0.005175 mol 3. Moles of KOH in sample = 0.005175 Concentration = moles/volume = 0.005175/0.025 = 0.207 mol -1 4. Molecular mass of KOH = 39 + 16 + 1 = 56 (g mol -1 ) 0.207 mol -1 KOH is 0.207 × 56 = 11.592 g -1 Quality control (page 194) Q9: Equation: ZnO + 2HCl → ZnCl 2 + H2 O Mole ratios: 1 mol ZnO reacts with 2 mol HCl Moles of HCl used: 0.500 × 0.02064 = 0.01032 mol Moles of ZnO: 0.01032/2 = 0.00516 mol GFM of ZnO: 65.4 + 16 = 81.4 0.00516 mol = 0.00516 × 81.4 = 0.42 g 0.42 g ml-1 ZnO = 420 g -1 (1 = 1000 ml) So the slurry contains 420 g ZnO -1 and is within requirements. The purity of aspirin tablets (page 194) Q10: Aspirin is a carboxylic acid (acetylsalicylic acid), so it can be estimated by titration with standard sodium hydroxide solution. © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 6 Q11: The formula of aspirin is: C 9 H8 O4 : RFM = 9×12 + 8×1 + 4×16 = 180 180 g aspirin is 1 mole 1 g aspirin is 1/180 mol 0.300 g aspirin (1 tablet) is 0.300/180 = 0.001667 mol Q12: From the equation 1 mol aspirin reacts with 1 mol NaOH So 0.001667 mol aspirin reacts with 0.001667 mol NaOH For the NaOH solution: 0.300 mol is contained in 1000 ml 1 mol is in 1000/0.300 ml 0.01667 mol is in (1000 × 0.001667)/0.300 = 5.556 ml Q13: • Taking one tablet is not representative, it could have a chip missing, for example. It would be better to take, say, ten, dissolve them in water and take an aliquot (part) of this solution for analysis. For example, you could make the solution accurately to 250 ml in a volumetric flask and take a 25 ml aliquot (1/10 th ) for analysis. • The titre of more than 50 ml is quite high. If you make a solution of several tablets (as above) you could take 1/20 th to get a better titre value. • This will also let you carry out a rough titration and as many accurate ones as you need to feel confident in the result. Back titration (page 196) Q14: 1. Moles of HCl remaining: m × v = 0.500 × 0.022 = 0.0110 mol 2. Moles of HCl originally: m × v = 5.00 × 0.005 = 0.0250 mol 3. Moles of HCl neutralised: 0.0250 - 0.0110 = 0.0140 mol 4. Balanced equation: CaCO 3 + 2HCl → CaCl2 + H2 O + CO2 5. Mole ratios: 1 mol CaCO 3 reacts with 2 mol HCl 6. 0.014 mol HCl reacts with 0.007 mol CaCO 3 7. Relative formula mass of CaCO 3: 40.0 + 12.0 + (3 × 16.0) = 100.0 8. 0.007 mol CaCO 3 is 0.7 g 9. One antacid tablet contains 0.7 g CaCO 3 . © H ERIOT-WATT U NIVERSITY 259 260 ANSWERS: TOPIC 6 The chloride ion content of seawater (precipitation) (page 197) Q15: • Na+ • Mg2+ • K+ • Ca2+ • Cl- • SO4 2- • HCO3 - Q16: Silver chromate(VI), AgCrO4 Q17: 1. Balanced equation: Ag + (aq) + Cl- (aq) → AgCl(s) 2. Mole ratios: 1 mol Ag+ reacts with 1 mol Cl3. Moles of Ag+ used: m × v = 0.50 × 0.012 = 0.006 mol 4. Moles of Cl- in 10 ml seawater = 0.006 mol 5. Relative formula mass of NaCl: 23.0 + 35.5 = 58.5 6. Mass of NaCl in 10 ml seawater: 0.006 × 58.5 = 0.351 g ≡ 35.1 g NaCl -1 End of Topic 6 test (page 199) Q18: The oil and aqueous solution will not mix so will be unable to react well. You might think of a way round this problem, before looking at the procedure (below) for a solution. Q19: 0.347424 g Q20: 6.28% Q21: 0.89 kg Q22: 0.009 moles Q23: 0.0045 mol l -1 Q24: 319.5 mg l -1 © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 7 261 7 End of unit test End of unit 3 test (page 202) Q1: b) decreases. Q2: b) endothermic. Q3: c) large. Q4: a) a small rise in temperature results in a large increase in reaction rate. Q5: b) 20 kJ mol-1 Q6: a) -30 kJ mol-1 Q7: a) Q8: d) London dispersion forces Q9: d) number of protons. Q10: c) bonds between molecules are weaker than bonds between ions. Q11: a) 4-methylpentan-2-one. Q12: b) ethanol and butanoic acid. Q13: b) 2-methylbut-2-ene and 2-methylbut-1-ene Q14: A: -398 kJ Q15: b) Decrease in temperature, increase in pressure Q16: 0.02 mol Q17: sulfur Q18: b) B Q19: 100cm3 Q20: Fe(s) Cl2(g) Heat Iced waterr FeCl3(g) © H ERIOT-WATT U NIVERSITY 262 ANSWERS: TOPIC 7 Q21: Polar covalent Q22: b) Ionic → polar covalent → pure covalent Q23: 190 kJ Q24: - 10 kJ Q25: decrease Q26: have no effect on Q27: + 26.5 kJ mol-1 Q28: - 32.75 kJ mol-1 Q29: Q30: Q31: Essential amino acids © H ERIOT-WATT U NIVERSITY ANSWERS: TOPIC 7 Q32: a) Q33: a) Q34: 156.4 kg Q35: A: ΔH1 + 2ΔH2 - ΔH3 Q36: 100 % Q37: Increasing pressure would move the equilibrium to the right (towards product), since there are fewer molecules on the product side of the equation. Q38: Increasing pressure would increase the yield. Q39: Decreasing the temperature would increase the yield since the forward reaction is exothermic and lowering the temperature would favour the reaction which produces heat. Q40: The lower pressure is cheaper to use and maintain and it is safer. Q41: None. Catalysts affect the rate of a reaction, not the position of the equilibrium. Q42: b) Sn2+ (aq) Q43: a) CH3 CH2 COCH3 → CH3 CH2 CH(OH)CH3 Q44: d) Zn + 2HCl → ZnCl 2 + H2 Q45: c) Iron(III) → iron(II) Q46: a) Fe + 2HCl → FeCl 2 + H2 Q47: Q48: Q49: a) OCl- + 2H+ + 2I- → Cl- + H2 O + I2 Q50: 0.02 mol l-1 © H ERIOT-WATT U NIVERSITY 263 264 ANSWERS: TOPIC 7 Q51: 1.03 mg l-1 Q52: a) Cl2 (g) + 2Br- (aq) → 2Cl- (aq) + Br2 (aq) and b) Br2 (l) + 2Fe2+ (aq) → 2Br- (aq) + 2Fe3+ (aq) Q53: a) 2IO3 - (aq) + 12H+ (aq) + 11e- → I2 (aq) + 6H2 O(l) Q54: H2 O2 (aq) + 2H+ (aq) + 2I- (aq) → 2H2 O(l) + I2 (aq) Q55: 0.0168 g Q56: As a general rule, award yourself a mark (up to a maximum of three marks in total) for each point you made. [NB this is a general rule only, remember there are no half marks awarded at Higher] • Measure the rate of reaction of the decomposition of hydrogen peroxide with manganese peroxide and therefore the faster the reaction, the higher the concentration of hydrogen peroxide. • You could also measure the conductivity as electrons are produced. The greater the number of electrons released the more hydrogen peroxide has decomposed. Therefore the higher the conductivity, the more hydrogen peroxide present in the gel. • You could measure the pH of the products as the lower the pH (higher H + concentration), the more hydrogen peroxide present in the gel. Q57: As a general rule, award yourself a mark (up to a maximum of three marks in total) for each point you made. [NB this is a general rule only, remember there are no half marks awarded at Higher] • If hydrogen peroxide was to leak from a rusty torpedo then it would decompose to form water and oxygen. • This reaction is exothermic and so releases energy in the form of heat. The oxygen gas which is released would combust with any fuel it came into contact with and this could cause an explosion. • The decomposition of hydrogen peroxide is slow at room temperature and pressure but the presence of the iron (in the rust) would act as a catalyst and greatly increase the rate of decomposition. © H ERIOT-WATT U NIVERSITY