Late Transition Metal Amido Complexes: Electronic
... about the M-N bonding, indicating a large polar -bond effect which results in a high anionic charge density on the nitrogen atom.[8] The negative charge can be effectively delocalized by NM -donation if vacant metal orbitals of suitable symmetry and energy are available, thus triggers a strengthe ...
... about the M-N bonding, indicating a large polar -bond effect which results in a high anionic charge density on the nitrogen atom.[8] The negative charge can be effectively delocalized by NM -donation if vacant metal orbitals of suitable symmetry and energy are available, thus triggers a strengthe ...
Sample Chapter 3
... activity from a tropical plant: what is its formula, and what quantity of metabolic products will establish a safe dosage level? Thousands of biologically active compounds have been found in plants and many are used in modern medicines. For example, in 1963, the U.S. Food and Drug Administration (FD ...
... activity from a tropical plant: what is its formula, and what quantity of metabolic products will establish a safe dosage level? Thousands of biologically active compounds have been found in plants and many are used in modern medicines. For example, in 1963, the U.S. Food and Drug Administration (FD ...
Topic 1 Quantitative Chemistry Answers - slider-dpchemistry-11
... Qualitative refers to the identity of a compound or element whereas quantitative refers to the amount of a known compound or element present in terms of mass, concentration or number of moles etc. 2. Define the following terms: a) element A substance that cannot be divided into simpler, smaller subs ...
... Qualitative refers to the identity of a compound or element whereas quantitative refers to the amount of a known compound or element present in terms of mass, concentration or number of moles etc. 2. Define the following terms: a) element A substance that cannot be divided into simpler, smaller subs ...
Calculations In Chemistry Modules 8 to 10
... The mole was originally defined by counting the number of atoms in one gram of hydrogen, the lightest atom. The definition has changed slightly over time. We now base our count on the isotope carbon-12 (exactly 12 grams of C-12 contains exactly one mole of C-12), but the original definition based on ...
... The mole was originally defined by counting the number of atoms in one gram of hydrogen, the lightest atom. The definition has changed slightly over time. We now base our count on the isotope carbon-12 (exactly 12 grams of C-12 contains exactly one mole of C-12), but the original definition based on ...
Spectroscopic Comparisons of the pH Dependencies of Fe
... where M signifies the bound metal ion. Although Fe- and Mn-specific SODs are very closely related to one another, they are essentially unrelated to the Cu and Zn-containing SODs. Fe- and Mn-SODs are dimers or tetramers of identical monomers, which each have one active site containing a single Fe or ...
... where M signifies the bound metal ion. Although Fe- and Mn-specific SODs are very closely related to one another, they are essentially unrelated to the Cu and Zn-containing SODs. Fe- and Mn-SODs are dimers or tetramers of identical monomers, which each have one active site containing a single Fe or ...
BSc Chemistry Syllabus - St. Xavier`s College
... (Prerequisites or topics for Self Study:- (i) Importance and application of qualitative and quantitative analysis (ii) Brief study of various methods of qualitative and quantitative analysis, (iii) Understanding reactions and reagents (iv) Understanding different types of bonds) 1. Quantitative Anal ...
... (Prerequisites or topics for Self Study:- (i) Importance and application of qualitative and quantitative analysis (ii) Brief study of various methods of qualitative and quantitative analysis, (iii) Understanding reactions and reagents (iv) Understanding different types of bonds) 1. Quantitative Anal ...
coordination of some monodentate and hybrid multident ate
... been prepared and characterised by a combination of *H, [P and 19F (as appropriate) NMR and IR spectroscopies and mass spectrometry. Some of these complexes have been isolated as single crystals and characterised by X-ray diffraction. The triarylphosphine ligands used in this study were P(4 -CH3 0 C ...
... been prepared and characterised by a combination of *H, [P and 19F (as appropriate) NMR and IR spectroscopies and mass spectrometry. Some of these complexes have been isolated as single crystals and characterised by X-ray diffraction. The triarylphosphine ligands used in this study were P(4 -CH3 0 C ...
The Impact of Ligand Design on the Coordination Chemistry and
... Second, for carbonylrhodium(I) complexes, (NNN)Rh(CO), substitution at the para-aryl positions predictably modulates the electronic properties and chemical reactivity. Oxidative addition reactions of the (NNN)Rh(CO) with iodoalkanes proceed about three orders of magnitude faster than those reported ...
... Second, for carbonylrhodium(I) complexes, (NNN)Rh(CO), substitution at the para-aryl positions predictably modulates the electronic properties and chemical reactivity. Oxidative addition reactions of the (NNN)Rh(CO) with iodoalkanes proceed about three orders of magnitude faster than those reported ...
Fundamental Equilibrium Concepts
... Imagine a beach populated with sunbathers and swimmers. As those basking in the sun get too hot and want to cool off, they head into the surf to swim. As the swimmers tire, they head to the beach to rest. If these two rates of transfer (sunbathers entering the water, swimmers leaving the water) are ...
... Imagine a beach populated with sunbathers and swimmers. As those basking in the sun get too hot and want to cool off, they head into the surf to swim. As the swimmers tire, they head to the beach to rest. If these two rates of transfer (sunbathers entering the water, swimmers leaving the water) are ...
mcdonald (pam78654) – HW 1: High School Concepts – laude
... mcdonald (pam78654) – HW 1: High School Concepts – laude – (89560) This print-out should have 40 questions. Multiple-choice questions may continue on the next column or page – find all choices before answering. 001 10.0 points Calculate the number of H2 O molecules in 1.00 cm3 of water at 0◦ C (dens ...
... mcdonald (pam78654) – HW 1: High School Concepts – laude – (89560) This print-out should have 40 questions. Multiple-choice questions may continue on the next column or page – find all choices before answering. 001 10.0 points Calculate the number of H2 O molecules in 1.00 cm3 of water at 0◦ C (dens ...
Part 3-ICHO-31-35
... The standard enthalpy of formation of CO2(g) and H2O(l) at 25.00 °C are –393.51 and –285.83 kJ mol-1, respectively. The gas constant, R = 8.314 J K-1 mol-1. (Relative atomic masses : H = 1.0; C = 12.0; O = 16.0) A sample of solid Q that weighs 0.6000 g, is combusted in an excess of oxygen in a bomb ...
... The standard enthalpy of formation of CO2(g) and H2O(l) at 25.00 °C are –393.51 and –285.83 kJ mol-1, respectively. The gas constant, R = 8.314 J K-1 mol-1. (Relative atomic masses : H = 1.0; C = 12.0; O = 16.0) A sample of solid Q that weighs 0.6000 g, is combusted in an excess of oxygen in a bomb ...
NAME NOTES: UNIT 8 THE MOLE AND STOICHIOMETRY (2
... that whatever you “put into the reaction”, you somehow, “get out” with products. A) How to Organize Your Conversion Factors 1) Every beginning to a stoichiometry problem should look like: ...
... that whatever you “put into the reaction”, you somehow, “get out” with products. A) How to Organize Your Conversion Factors 1) Every beginning to a stoichiometry problem should look like: ...
Stoichiometric Calculations
... · In terms of liters, a total of 4 liters of gas react to form 2 liters of gas (at STP, 89.2 L of gas react to yield 44.8 L). The number of moles, particles and liters before the arrow is not the same as after the arrow. Therefore, these quantities are not conserved in a balanced equation. ...
... · In terms of liters, a total of 4 liters of gas react to form 2 liters of gas (at STP, 89.2 L of gas react to yield 44.8 L). The number of moles, particles and liters before the arrow is not the same as after the arrow. Therefore, these quantities are not conserved in a balanced equation. ...
Solid-State 23Na Nuclear Magnetic Resonance of Sodium
... relatively high magnetogyric ratio, γ ) 7.0761 × 107 rad‚s-1‚T-1. However, the fact that 23Na is also a quadrupolar nucleus (I ) 3/2 and Q ) 0.1 × 10-28 m2) imposes some practical difficulties not only on carrying out NMR experiments, but also on interpreting the experimental data. One of the major ...
... relatively high magnetogyric ratio, γ ) 7.0761 × 107 rad‚s-1‚T-1. However, the fact that 23Na is also a quadrupolar nucleus (I ) 3/2 and Q ) 0.1 × 10-28 m2) imposes some practical difficulties not only on carrying out NMR experiments, but also on interpreting the experimental data. One of the major ...
Chapter 3 Solutions - Bremerton School District
... There are three peaks in the mass spectrum, each 2 mass units apart. This is consistent with two isotopes, differing in mass by two mass units. The peak at 157.84 corresponds to a Br2 molecule composed of two atoms of the lighter isotope. This isotope has mass equal to 157.84/2 or 78.92. This corres ...
... There are three peaks in the mass spectrum, each 2 mass units apart. This is consistent with two isotopes, differing in mass by two mass units. The peak at 157.84 corresponds to a Br2 molecule composed of two atoms of the lighter isotope. This isotope has mass equal to 157.84/2 or 78.92. This corres ...
chemistry - University of Malaya
... Perform chemistry laboratory procedures, to solve problems, to record and to analyze data and to present experimental results effectively. Demonstrate social expertise for environmental sustainable development in the practice of chemistry and management of the flow of activities and tasks with the h ...
... Perform chemistry laboratory procedures, to solve problems, to record and to analyze data and to present experimental results effectively. Demonstrate social expertise for environmental sustainable development in the practice of chemistry and management of the flow of activities and tasks with the h ...
i̇zmi̇r institute of technology graduate school of engineering
... Manipulation and Characterization Techniques for Air Sensitive Compounds Selected Topics in Inorganic Chemistry Spectroscopic Methods in Inorganic Chemistry Synthetic Methods in Coordination Chemistry Principles of Asymmetric Synthesis Reactions and Synthesis in Organic Chemistry Selected T ...
... Manipulation and Characterization Techniques for Air Sensitive Compounds Selected Topics in Inorganic Chemistry Spectroscopic Methods in Inorganic Chemistry Synthetic Methods in Coordination Chemistry Principles of Asymmetric Synthesis Reactions and Synthesis in Organic Chemistry Selected T ...
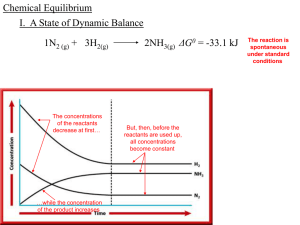
Chemical Equilibrium - local.brookings.k12.sd.us
... and _________ ___________________, Chemical Equilibrium which states, at a given ___________, temperature a chemical state in which a system may reach a _____ ratio of _______ reactant and particular _____ product ____________ concentrations has a constant _______ _______ value Cato Maximilian Guldb ...
... and _________ ___________________, Chemical Equilibrium which states, at a given ___________, temperature a chemical state in which a system may reach a _____ ratio of _______ reactant and particular _____ product ____________ concentrations has a constant _______ _______ value Cato Maximilian Guldb ...
(NH3)n and NH2 - Sanov Group
... lowest binding energy transition (peaking at 0.77 eV) is assigned to removal of an electron from the b1 HOMO of NH2 − to generate the neutral amidogen radical in its 2 B1 ground state, as previously identified.30, 39 The narrow width of this transition (labeled 2 B1 in Figure 3) and lack of vibratio ...
... lowest binding energy transition (peaking at 0.77 eV) is assigned to removal of an electron from the b1 HOMO of NH2 − to generate the neutral amidogen radical in its 2 B1 ground state, as previously identified.30, 39 The narrow width of this transition (labeled 2 B1 in Figure 3) and lack of vibratio ...
Stoichiometry Chapter 3 CHEMA1301 [Compatibility Mode]
... of increasing numbers of carbon atoms: 12 g 12C, 1 mol C2H2, 9*1023 molecules of CO2. 12 g of 12C contains 1 mol of C atoms, that is 6.02*1023 C atoms. One mol of C2H2 contains 2*6.02*1023 C atoms. Because there are two C atoms in each molecule. Because each CO2 molecule contains one C atom, the CO2 ...
... of increasing numbers of carbon atoms: 12 g 12C, 1 mol C2H2, 9*1023 molecules of CO2. 12 g of 12C contains 1 mol of C atoms, that is 6.02*1023 C atoms. One mol of C2H2 contains 2*6.02*1023 C atoms. Because there are two C atoms in each molecule. Because each CO2 molecule contains one C atom, the CO2 ...
Get cached PDF
... The use of chiral lanthanide complexes as probes to investigate interactions with other chiral molecules and macromolecules is of particular interest. A key step in the development of such systems is the preparation of a single, rigid enantiomer of the complex which is conformationally rigid on the ...
... The use of chiral lanthanide complexes as probes to investigate interactions with other chiral molecules and macromolecules is of particular interest. A key step in the development of such systems is the preparation of a single, rigid enantiomer of the complex which is conformationally rigid on the ...
Chapter 15: Chemical Equilibrium
... When this equilibrium state is achieved, the concentrations of all the species in solution are constant, even though the forward and reverse reactions continue to take place. Note that when a system is at equilibrium, while the rates of the forward and reverse reactions are equal, the rate constants ...
... When this equilibrium state is achieved, the concentrations of all the species in solution are constant, even though the forward and reverse reactions continue to take place. Note that when a system is at equilibrium, while the rates of the forward and reverse reactions are equal, the rate constants ...
Chemistry
... Behaviour of real gases: Deviations from ideal gas behaviour, compressibility factor, Z, and its variation with pressure and temperature for different gases. Causes of deviation from ideal behaviour. van der Waals equation of state, its derivation and application in explaining real gas behaviour, ca ...
... Behaviour of real gases: Deviations from ideal gas behaviour, compressibility factor, Z, and its variation with pressure and temperature for different gases. Causes of deviation from ideal behaviour. van der Waals equation of state, its derivation and application in explaining real gas behaviour, ca ...
Chapter 18 pdf
... Waage proposed the law of chemical equilibrium, which states that at a given temperature, a chemical system may reach a state in which a particular ratio of reactant and product concentrations has a constant value. For example, the general equation for a reaction at equilibrium can be written as fol ...
... Waage proposed the law of chemical equilibrium, which states that at a given temperature, a chemical system may reach a state in which a particular ratio of reactant and product concentrations has a constant value. For example, the general equation for a reaction at equilibrium can be written as fol ...
Host–guest chemistry

In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest chemistry encompasses the idea of molecular recognition and interactions through noncovalent bonding. Noncovalent bonding is critical in maintaining the 3D structure of large molecules, such as proteins and is involved in many biological processes in which large molecules bind specifically but transiently to one another. There are four commonly mentioned types of non-covalent interactions: hydrogen bonds, ionic bonds, van der Waals forces, and hydrophobic interactions.














![Stoichiometry Chapter 3 CHEMA1301 [Compatibility Mode]](http://s1.studyres.com/store/data/014247793_1-84b4b6fe6fa37d77afbf7eb657ee347a-300x300.png)