National 5 Chemistry Prelim Revision 1
... Which of the following compounds belongs to the same homologous series as the compound with the molecular formula C3H8? ...
... Which of the following compounds belongs to the same homologous series as the compound with the molecular formula C3H8? ...
Unit 5 Objectives
... Unit 5 – Counting Particles - Objectives Review Concepts a. Types of substances b. Chemical formulas of substances (U 4) 1. State evidence for Avogadro’s Hypothesis. Use Avogadro’s Hypothesis and experimental data to determine the relative mass of molecules. ...
... Unit 5 – Counting Particles - Objectives Review Concepts a. Types of substances b. Chemical formulas of substances (U 4) 1. State evidence for Avogadro’s Hypothesis. Use Avogadro’s Hypothesis and experimental data to determine the relative mass of molecules. ...
Chemical Equations and Reactions notes File
... total mass of reactants = total mass of products ...
... total mass of reactants = total mass of products ...
Vocabulary: "Chemical Bonding"
... Search the pages of Chapter 7 and anywhere else in your text or on the internet and write the definitions for each of the following terms. ...
... Search the pages of Chapter 7 and anywhere else in your text or on the internet and write the definitions for each of the following terms. ...
Moles Practice Test
... 9. Narcotics agents confiscated an unknown substance that was suspected to be methaqualone. Its molecular formula is C16H14N2O. Analysis found the sample to have 77% carbon, 5.5% hydrogen, 15.3% nitrogen, and the rest oxygen. Was it methaqualone? ...
... 9. Narcotics agents confiscated an unknown substance that was suspected to be methaqualone. Its molecular formula is C16H14N2O. Analysis found the sample to have 77% carbon, 5.5% hydrogen, 15.3% nitrogen, and the rest oxygen. Was it methaqualone? ...
Moles Practice Test
... 1. 16.0 g/mol 2. 164.1 g/mol 3. 71.8 g/mol 4. 1.05 moles 5. 40.g 6a. 6.56 x 1024 molecules 6b. 3.94 x 1025 atoms O 7. 234g 8. 2.73 x 1024 9. 58.5% oxygen 10. No 11. 469g 12. CO 13. FeCl3 14. C4H8O8 15. empirical = Fe2O3 name = iron(III) oxide 70% Fe, 30% O 16. C8H10N4O2 ...
... 1. 16.0 g/mol 2. 164.1 g/mol 3. 71.8 g/mol 4. 1.05 moles 5. 40.g 6a. 6.56 x 1024 molecules 6b. 3.94 x 1025 atoms O 7. 234g 8. 2.73 x 1024 9. 58.5% oxygen 10. No 11. 469g 12. CO 13. FeCl3 14. C4H8O8 15. empirical = Fe2O3 name = iron(III) oxide 70% Fe, 30% O 16. C8H10N4O2 ...
Moles Practice Test
... 10. Narcotics agents confiscated an unknown substance that was suspected to be methaqualone. Its molecular formula is C16H14N2O. Analysis found the sample to have 77% carbon, 5.5% hydrogen, 15.3% nitrogen, and the rest oxygen. Was it methaqualone? ...
... 10. Narcotics agents confiscated an unknown substance that was suspected to be methaqualone. Its molecular formula is C16H14N2O. Analysis found the sample to have 77% carbon, 5.5% hydrogen, 15.3% nitrogen, and the rest oxygen. Was it methaqualone? ...
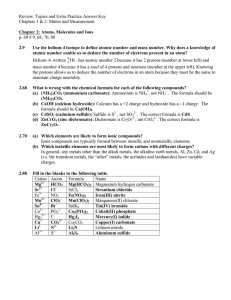
HW 2-1 Review Chap 2 Key
... Use the helium-4 isotope to define atomic number and mass number. Why does a knowledge of atomic number enable us to deduce the number of electrons present in an atom? Helium–4, written 42 He , has atomic number 2 because it has 2 protons (number at lower left) and mass number 4 because it has a tot ...
... Use the helium-4 isotope to define atomic number and mass number. Why does a knowledge of atomic number enable us to deduce the number of electrons present in an atom? Helium–4, written 42 He , has atomic number 2 because it has 2 protons (number at lower left) and mass number 4 because it has a tot ...
Host–guest chemistry

In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest chemistry encompasses the idea of molecular recognition and interactions through noncovalent bonding. Noncovalent bonding is critical in maintaining the 3D structure of large molecules, such as proteins and is involved in many biological processes in which large molecules bind specifically but transiently to one another. There are four commonly mentioned types of non-covalent interactions: hydrogen bonds, ionic bonds, van der Waals forces, and hydrophobic interactions.