structure and thermodynamics of lanthanide
... sphere20. This model of solvent separated ion pairing was supported by studies of the spectra of Nd(iii) which showed• no change up to concentrations of 5M chloride ion2 1 A spectral change was observed at higher concentrations which may be related to a change in coordination number from nine to eig ...
... sphere20. This model of solvent separated ion pairing was supported by studies of the spectra of Nd(iii) which showed• no change up to concentrations of 5M chloride ion2 1 A spectral change was observed at higher concentrations which may be related to a change in coordination number from nine to eig ...
20. Chemical Equilibrium
... In most of the chemical reactions we have studied so far, it appears as though all of the reactants are converted to products before a reaction stops. In truth, however, experiments show that the conversion of reactants into products is often incomplete in chemical reactions. This is the case no mat ...
... In most of the chemical reactions we have studied so far, it appears as though all of the reactants are converted to products before a reaction stops. In truth, however, experiments show that the conversion of reactants into products is often incomplete in chemical reactions. This is the case no mat ...
Stoichiometry - coercingmolecules
... Chang, R. 2002. Chemistry 7th ed. Singapore: McGraw-Hill. Silberberg, M. 2010. Principles of General Chemistry. 2nd ed. New York: McGraw-Hill. ...
... Chang, R. 2002. Chemistry 7th ed. Singapore: McGraw-Hill. Silberberg, M. 2010. Principles of General Chemistry. 2nd ed. New York: McGraw-Hill. ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... For molecules! Avogadro’s number represents the number of particles, whether atoms or molecules. Eg. Calculate the number of molecules in 4.99mol of methane. 4.99 mol x 6.022 x 1023 molecules = 3.00 x 1024 molecules mol of CH4 Stoichiometry ...
... For molecules! Avogadro’s number represents the number of particles, whether atoms or molecules. Eg. Calculate the number of molecules in 4.99mol of methane. 4.99 mol x 6.022 x 1023 molecules = 3.00 x 1024 molecules mol of CH4 Stoichiometry ...
COURSES SCHEME & SYLLABUS
... Derivation of Debye-Huckel limiting law. Classical Thermodynamics: Concepts involved in first, second and third law of thermodynamic, Free energy and entropy of mixing, Partial molar quantities, Gibbs-Duhem equation. Equilibrium constant, Temperature-dependence of equilibrium constant, Thermodynamic ...
... Derivation of Debye-Huckel limiting law. Classical Thermodynamics: Concepts involved in first, second and third law of thermodynamic, Free energy and entropy of mixing, Partial molar quantities, Gibbs-Duhem equation. Equilibrium constant, Temperature-dependence of equilibrium constant, Thermodynamic ...
Chemistry: Percent Yield
... proportion. A chemical compound can be broken down by chemical means. A chemical compound can be represented by a specific chemical formula and assigned a name based on the IUPAC system. 35: 3.3f The percent composition by mass of each element in a compound can be calculated mathematically 37: 3.3iv ...
... proportion. A chemical compound can be broken down by chemical means. A chemical compound can be represented by a specific chemical formula and assigned a name based on the IUPAC system. 35: 3.3f The percent composition by mass of each element in a compound can be calculated mathematically 37: 3.3iv ...
unit-4-notes-1_enthalpy-and-entropy
... 2. Letting material into or out of the system will affect rates so a system at equilibrium is a closed system. 3. Again, consider the equilibrium reaction: N2O4 2 NO2 In the example that we did to construct the graphs, we had started with pure N2O4 and no NO2. The forward reaction rate was high at t ...
... 2. Letting material into or out of the system will affect rates so a system at equilibrium is a closed system. 3. Again, consider the equilibrium reaction: N2O4 2 NO2 In the example that we did to construct the graphs, we had started with pure N2O4 and no NO2. The forward reaction rate was high at t ...
Chapter
... Types of Formula Structural Formula • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess a ...
... Types of Formula Structural Formula • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess a ...
Chapter
... Types of Formula Structural Formula • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess a ...
... Types of Formula Structural Formula • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess a ...
Predictive thermodynamics for ionic solids and
... played a significant role in yielding the thermodynamics. In essence, VBT [together with its isomegethic (‘‘equal size’’) rule,24 which vastly extends its application to new and hypothesised materials] relates the formula unit volumes, Vm, of materials, however measured or estimated, to their thermo ...
... played a significant role in yielding the thermodynamics. In essence, VBT [together with its isomegethic (‘‘equal size’’) rule,24 which vastly extends its application to new and hypothesised materials] relates the formula unit volumes, Vm, of materials, however measured or estimated, to their thermo ...
The Citrate Carrier CitS Probed by Single
... citS gene encodes a highly hydrophobic integral membrane protein with a postulated topology of 11 transmembrane helices connected by hydrophilic loops (van Geest et al., 1999). CitS catalyzes the highly specific, electroneutral uptake of Hcitrate2- in cotransport with two Naþ ions (Lolkema et al., 19 ...
... citS gene encodes a highly hydrophobic integral membrane protein with a postulated topology of 11 transmembrane helices connected by hydrophilic loops (van Geest et al., 1999). CitS catalyzes the highly specific, electroneutral uptake of Hcitrate2- in cotransport with two Naþ ions (Lolkema et al., 19 ...
ppt
... Create and complete an ICE table for an equilibrium system. Draw graphs of c vs. t to illustrate a chemical system approaching equilibrium. Use appropriate terminology such as dynamic equilibrium, reversible reaction, closed system, equilibrium concentrations, phase equilibrium, solubility equilibri ...
... Create and complete an ICE table for an equilibrium system. Draw graphs of c vs. t to illustrate a chemical system approaching equilibrium. Use appropriate terminology such as dynamic equilibrium, reversible reaction, closed system, equilibrium concentrations, phase equilibrium, solubility equilibri ...
ADSORPTION OF Cr(NH3)6 3+ AND Cr(en)3 3+ ON CLAY
... increase noted for Cr(en)33+ with all three clays indicates reactions other than simple adsorption. For chlorite and illite, the "equilibrium" pH for both complexes was greater than that for the control sample. On the other hand, the pHs of the chromium complex-kaolinite solutions were equivalent to ...
... increase noted for Cr(en)33+ with all three clays indicates reactions other than simple adsorption. For chlorite and illite, the "equilibrium" pH for both complexes was greater than that for the control sample. On the other hand, the pHs of the chromium complex-kaolinite solutions were equivalent to ...
Chapter 3 - Educator
... Once we know the formulas of the reactants and products in a reaction, we can write the unbalanced equation. We then balance the equation by determining the coefficients that provide equal numbers of each type of atom on each side of the equation. For most purposes, a balanced equation should contai ...
... Once we know the formulas of the reactants and products in a reaction, we can write the unbalanced equation. We then balance the equation by determining the coefficients that provide equal numbers of each type of atom on each side of the equation. For most purposes, a balanced equation should contai ...
Stoichiometry: Calculations with Chemical Formulas and
... Which is the Limiting Reagent? Step 1: #moles CHCl3 = mass/GFM = 15.9 g/119.5 g/mol = 0.133 mol #moles Cl2 = mass/GFM = 12.6 g/71.0 g/mol = 0.177 mol Step 2: Use these equations: # mol reactant CHCl3 / coefficient CHCl3= 0.133/ 2 = 0.0665 # mol reactant Cl2 / coefficent Cl2 = 0.177/ 2 = 0.0885 Step ...
... Which is the Limiting Reagent? Step 1: #moles CHCl3 = mass/GFM = 15.9 g/119.5 g/mol = 0.133 mol #moles Cl2 = mass/GFM = 12.6 g/71.0 g/mol = 0.177 mol Step 2: Use these equations: # mol reactant CHCl3 / coefficient CHCl3= 0.133/ 2 = 0.0665 # mol reactant Cl2 / coefficent Cl2 = 0.177/ 2 = 0.0885 Step ...
Some basic concepts of chemistry
... The masses of oxygen which combine with same mass of hydrogen in these two compounds bear a simple ratio 1 : 2. Law of reciprocal proportions: This law was given by Richter in 1794. Statement: It states that, “When two different elements combine separately with the same weight of a third element, th ...
... The masses of oxygen which combine with same mass of hydrogen in these two compounds bear a simple ratio 1 : 2. Law of reciprocal proportions: This law was given by Richter in 1794. Statement: It states that, “When two different elements combine separately with the same weight of a third element, th ...
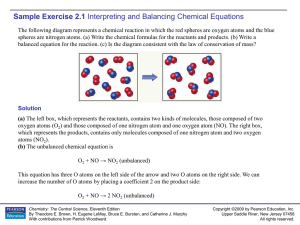
Sample Exercise 2.1
... 12 g 12C, 1 mol C2H2, 9 1023 molecules of CO2. Solution Analyze We are given amounts of different substances expressed in grams, moles, and number of molecules and asked to arrange the samples in order of increasing numbers of C atoms. Plan To determine the number of C atoms in each sample, we mus ...
... 12 g 12C, 1 mol C2H2, 9 1023 molecules of CO2. Solution Analyze We are given amounts of different substances expressed in grams, moles, and number of molecules and asked to arrange the samples in order of increasing numbers of C atoms. Plan To determine the number of C atoms in each sample, we mus ...
Single-Molecule Fluorescence Resonance Energy Transfer
... from free dyes and unlabeled nucleic acids by using sizeexclusion gel filtration, HPLC, or gel electrophoresis. How Are Dye Molecules Attached to Protein? It is commonplace to label proteins and antibodies with amine-reactive dyes because lysine residues are frequently found on protein surfaces. But ...
... from free dyes and unlabeled nucleic acids by using sizeexclusion gel filtration, HPLC, or gel electrophoresis. How Are Dye Molecules Attached to Protein? It is commonplace to label proteins and antibodies with amine-reactive dyes because lysine residues are frequently found on protein surfaces. But ...
Chapter - Imperial Valley College
... Types of Formula Structural Formula • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess a ...
... Types of Formula Structural Formula • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess a ...
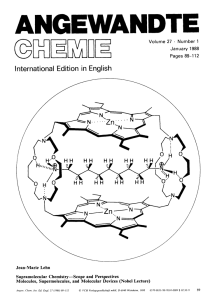
Supramolecular Chemistry—Scope and Perspectives Molecules
... coordination. Supramolecular catalysis by receptors bearing reactive groups effects bond cleavage reactions as well as synthetic bond formation via cocatalysis. Lipophilic receptor molecules act as selective carriers for various substrates and make it possible to set up coupled transport processes l ...
... coordination. Supramolecular catalysis by receptors bearing reactive groups effects bond cleavage reactions as well as synthetic bond formation via cocatalysis. Lipophilic receptor molecules act as selective carriers for various substrates and make it possible to set up coupled transport processes l ...
Naming Compounds - Kowenscience.com
... • NON-Polar bonds – Electrons shared evenly in the bond – E-neg difference is zero ...
... • NON-Polar bonds – Electrons shared evenly in the bond – E-neg difference is zero ...
Click Chemistry in Peptide-Based Drug Design
... peptides [1]. Many strategies have been used to increase the stability of peptide drug candidates, such as introducing non-natural amino acids, terminal protection, cyclization, and backbone modification. Backbone modifications, such as NH-amide alkylation, replacement of the carbonyl function of th ...
... peptides [1]. Many strategies have been used to increase the stability of peptide drug candidates, such as introducing non-natural amino acids, terminal protection, cyclization, and backbone modification. Backbone modifications, such as NH-amide alkylation, replacement of the carbonyl function of th ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • Stoichiometric factors (or molar ratios) may be used to convert between quantities of reactants and products in a reaction. • It is important to realize that the stoichiometric ratios are the ideal proportions in which reactants are needed to form products. Stoichiometry © 2009, Prentice-Hall, Inc ...
... • Stoichiometric factors (or molar ratios) may be used to convert between quantities of reactants and products in a reaction. • It is important to realize that the stoichiometric ratios are the ideal proportions in which reactants are needed to form products. Stoichiometry © 2009, Prentice-Hall, Inc ...
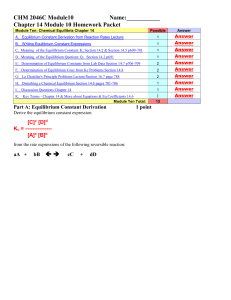
CHEM 1212 Module Ten-Chapter 16 Name
... remain constant with time , it is said that the system has reached a state of ___________equilibrium. __________________ 4. The _______ _________ Q is obtained by by applying the law of mass action using the initial concentrations of both the reactants and the products instead of the equilibrium con ...
... remain constant with time , it is said that the system has reached a state of ___________equilibrium. __________________ 4. The _______ _________ Q is obtained by by applying the law of mass action using the initial concentrations of both the reactants and the products instead of the equilibrium con ...
Complexometric Titration
... bonds and produce coordination compound or complexes. Molecule or ion with at least 1 pair of unshared electron can form covalent bond with metal ion = ligands The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs Eg of ligands = ammoni ...
... bonds and produce coordination compound or complexes. Molecule or ion with at least 1 pair of unshared electron can form covalent bond with metal ion = ligands The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs Eg of ligands = ammoni ...
Host–guest chemistry

In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest chemistry encompasses the idea of molecular recognition and interactions through noncovalent bonding. Noncovalent bonding is critical in maintaining the 3D structure of large molecules, such as proteins and is involved in many biological processes in which large molecules bind specifically but transiently to one another. There are four commonly mentioned types of non-covalent interactions: hydrogen bonds, ionic bonds, van der Waals forces, and hydrophobic interactions.