Amino acid residues that determine functional specificity of NADP
... Apart from 344Lys, 345Tyr, and 351Val, several other amino acids contact cofactor in most considered structures: substrate-specific residues 103Leu, 105Thr, 337Ala, and 341Thr contact the nicotinamide nucleotide and thus spatially lie between the cofactor-binding and the substratebinding pockets. The ...
... Apart from 344Lys, 345Tyr, and 351Val, several other amino acids contact cofactor in most considered structures: substrate-specific residues 103Leu, 105Thr, 337Ala, and 341Thr contact the nicotinamide nucleotide and thus spatially lie between the cofactor-binding and the substratebinding pockets. The ...
1- Glycolysis
... cycle or the Krebs cycle: is a series of chemical reactions used by all aerobic organisms to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate, (ATP.) In addition, the ...
... cycle or the Krebs cycle: is a series of chemical reactions used by all aerobic organisms to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate, (ATP.) In addition, the ...
Biocatalysis - School of Chemical Sciences
... conditions, are also very attractive in commercial applications. The characteristics of limited operating regions, substrate or product inhibition, and reactions in aqueous solutions have often been considered as the most serious drawbacks of biocatalysts. Many of these drawbacks, however, turn out ...
... conditions, are also very attractive in commercial applications. The characteristics of limited operating regions, substrate or product inhibition, and reactions in aqueous solutions have often been considered as the most serious drawbacks of biocatalysts. Many of these drawbacks, however, turn out ...
Vitamins
... ketoglutarate, which plays a key role in energy metabolism of most cells, is particularly important in tissues of the nervous system. In thiamine deficiency, the activity of these two dehydrogenase reactions is decreased, resulting in a decreased production of ATP and, thus, impaired cellular func ...
... ketoglutarate, which plays a key role in energy metabolism of most cells, is particularly important in tissues of the nervous system. In thiamine deficiency, the activity of these two dehydrogenase reactions is decreased, resulting in a decreased production of ATP and, thus, impaired cellular func ...
Chapter 16 The Citric Acid Cycle
... • A 2-carbon unit Acetyl-CoA is added to the cycle • And two CO2 molecules leave (but they are different carbons…) • During the course of changes in the carbon skeleton and its oxidation state • And the transfer of energy to form GTP (aka. the “Canadian $”) and reducing power, as NADH and FADH2 • It ...
... • A 2-carbon unit Acetyl-CoA is added to the cycle • And two CO2 molecules leave (but they are different carbons…) • During the course of changes in the carbon skeleton and its oxidation state • And the transfer of energy to form GTP (aka. the “Canadian $”) and reducing power, as NADH and FADH2 • It ...
Medical Biochemistry Review #2 By
... The TCA cycle showing enzymes, substrates and products. The abbreviated enzymes are: IDH = isocitrate dehydrogenase and a-KGDH = a-ketoglutarate dehydrogenase. The GTP generated during the succinate thiokinase (succinyl-CoA synthetase) reaction is equivalent to a mole of ATP by virtue of the presen ...
... The TCA cycle showing enzymes, substrates and products. The abbreviated enzymes are: IDH = isocitrate dehydrogenase and a-KGDH = a-ketoglutarate dehydrogenase. The GTP generated during the succinate thiokinase (succinyl-CoA synthetase) reaction is equivalent to a mole of ATP by virtue of the presen ...
Fatty acid catabolism leture2-3
... This condition is called “acidosis” which can lead to com or death. High concentration of ketone bodies in blood and urine is referred as “ketosis”. Due to high concentration of acetoacetate, which is converted to acetone, the breath and urine of theuntreated diabetic ...
... This condition is called “acidosis” which can lead to com or death. High concentration of ketone bodies in blood and urine is referred as “ketosis”. Due to high concentration of acetoacetate, which is converted to acetone, the breath and urine of theuntreated diabetic ...
LABORATORY DIAGNOSIS IN LIVER DISEASES 24.48 MB
... Most drugs are metabolized by the liver. Low substrate specificity of some hepatic enzymes produces a wide-ranging capability for drug metabolism Hepatic metabolism usually increases the hydrophilicity of drugs and therefore their ability to be excreted. Metabolites produced are less pharmacological ...
... Most drugs are metabolized by the liver. Low substrate specificity of some hepatic enzymes produces a wide-ranging capability for drug metabolism Hepatic metabolism usually increases the hydrophilicity of drugs and therefore their ability to be excreted. Metabolites produced are less pharmacological ...
Transport of Ammonia to the liver
... The reactions of the transaminases are reversible and the equilibrium constant is around (1) which means that they can go both ways depending on the concentration. Glutamate dehydrogenase which removes the amino group from the glutamate behaves the same way, it's reversible, it can give glutamate a ...
... The reactions of the transaminases are reversible and the equilibrium constant is around (1) which means that they can go both ways depending on the concentration. Glutamate dehydrogenase which removes the amino group from the glutamate behaves the same way, it's reversible, it can give glutamate a ...
Evolutionary algorithm for metabolic pathways synthesis - FICH-UNL
... model a metabolic pathway, where each gene is a reaction. Starting from a set of candidate networks, metabolic pathways are combined together to produce new potential solutions. The searching process is guided by an objetive function, which takes values in [0, 1] range, that assesses four aspects of ...
... model a metabolic pathway, where each gene is a reaction. Starting from a set of candidate networks, metabolic pathways are combined together to produce new potential solutions. The searching process is guided by an objetive function, which takes values in [0, 1] range, that assesses four aspects of ...
Intended Use
... Samples with values above 500 IU/L should be diluted 1:1 with saline, re-assayed and the results multiplied by two. Patients with severe vitamin B6 deficiency could have a decreased recovery of AST, presumably due to a lack of pyridoxal phosphate.13 ...
... Samples with values above 500 IU/L should be diluted 1:1 with saline, re-assayed and the results multiplied by two. Patients with severe vitamin B6 deficiency could have a decreased recovery of AST, presumably due to a lack of pyridoxal phosphate.13 ...
electron transport chain
... This process produces some ATP and carbon dioxide in the mitochondrion. This process uses energy captured from electrons flowing to oxygen to produce most of the ATPs in cellular respiration. This process converts pyruvic acid to acetyl CoA. This process splits glucose in half and produces 2 ATPs fo ...
... This process produces some ATP and carbon dioxide in the mitochondrion. This process uses energy captured from electrons flowing to oxygen to produce most of the ATPs in cellular respiration. This process converts pyruvic acid to acetyl CoA. This process splits glucose in half and produces 2 ATPs fo ...
Chapter 13 Carbohydrate Metabolism
... FADH2, which are necessary for the reduction of oxygen and ATP synthesis in the electron transport chain. – The citric acid cycle also functions as a source of intermediates for biosynthesis of other important molecules (e.g., some amino acids). • The reactions of the citric acid cycle occur within ...
... FADH2, which are necessary for the reduction of oxygen and ATP synthesis in the electron transport chain. – The citric acid cycle also functions as a source of intermediates for biosynthesis of other important molecules (e.g., some amino acids). • The reactions of the citric acid cycle occur within ...
OXIDATIVE PHOSPHORYLATION
... • Protons transported from the matrix to the inner mitochondrial space results in an electric gradient and a pH gradient • As the protons flow through the membrane channel back into the matrix they drive ATP synthesis Occurs with energy utilized by ATP synthase This proton transport couples electron ...
... • Protons transported from the matrix to the inner mitochondrial space results in an electric gradient and a pH gradient • As the protons flow through the membrane channel back into the matrix they drive ATP synthesis Occurs with energy utilized by ATP synthase This proton transport couples electron ...
NMEICT PROJECT
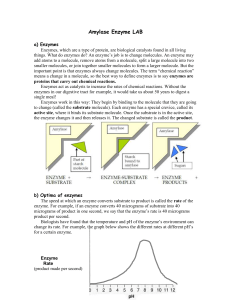
... in understanding the rates of reactions which assist in judging the kind of reaction that enzyme follows. (single- or multiple-substrate mechanism). Enzyme activity measures the amount of enzyme in a reaction. There are two methods to measure enzyme activity: loss of substrate and formation of produ ...
... in understanding the rates of reactions which assist in judging the kind of reaction that enzyme follows. (single- or multiple-substrate mechanism). Enzyme activity measures the amount of enzyme in a reaction. There are two methods to measure enzyme activity: loss of substrate and formation of produ ...
Gluconeogenesis: Objectives
... i. Gluconeogenesis occurs mainly in the liver (90%) and also in the kidneys c. What parts of the cell participate in gluconeogenesis? i. In the mitochondria and the cytoplasm d. Name the most common precursors for gluconeogenesis. i. Lactate from exercising muscle and red blood cells ii. The breakdo ...
... i. Gluconeogenesis occurs mainly in the liver (90%) and also in the kidneys c. What parts of the cell participate in gluconeogenesis? i. In the mitochondria and the cytoplasm d. Name the most common precursors for gluconeogenesis. i. Lactate from exercising muscle and red blood cells ii. The breakdo ...
CoA
... a) fatty acid from which they are derived; b) specific functions of each eicosanoid; c) general pathway of production; effects of glucocorticoids (cortisol) and aspirin ...
... a) fatty acid from which they are derived; b) specific functions of each eicosanoid; c) general pathway of production; effects of glucocorticoids (cortisol) and aspirin ...
ENERGY CURRENCY
... converted into ATP. In order for this conversion to occur, oxidative pathways must be available. NAD+ is nicotinamide adenine dinucleotide and is found in all cells. It is actually classified as a coenzyme . In its reduced high energy form it is officially NADH + H+. (In this discussion, it will be ...
... converted into ATP. In order for this conversion to occur, oxidative pathways must be available. NAD+ is nicotinamide adenine dinucleotide and is found in all cells. It is actually classified as a coenzyme . In its reduced high energy form it is officially NADH + H+. (In this discussion, it will be ...
Biosynthesis of Amino Acids
... Arginine biosynthesis: • In mammals arginine is synthesized from glutamate via urea cycle. • In bacteria, arginine is synthesized from glutamate in pathway different from urea cycle in mammals because most bacteria do not have arginase; not form ornithine from arginine . • Ornithine could also be s ...
... Arginine biosynthesis: • In mammals arginine is synthesized from glutamate via urea cycle. • In bacteria, arginine is synthesized from glutamate in pathway different from urea cycle in mammals because most bacteria do not have arginase; not form ornithine from arginine . • Ornithine could also be s ...
Glycolysis Worksheet High School Biology Use the
... a)The transfer of an inorganic phosphate group (Pi) from one substrate to another by way of an enzyme b) the addition of a phosphate (PO43−) group to a protein or other organic molecule c) the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion. d)metabolic pathway in whic ...
... a)The transfer of an inorganic phosphate group (Pi) from one substrate to another by way of an enzyme b) the addition of a phosphate (PO43−) group to a protein or other organic molecule c) the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion. d)metabolic pathway in whic ...
Chapter 2 - Water - Technicalsymposium
... leucine R= 4 carbon branched side chain isoleucine R = 2 chiral centers proline R = ring; puts bends or kinks in proteins; contains a secondary amino group 2) aromatic (R groups have phenyl ring) phenylalanine - very hydrophobic tyrosine - hydrophobic, but not as much because of polar groups tryptop ...
... leucine R= 4 carbon branched side chain isoleucine R = 2 chiral centers proline R = ring; puts bends or kinks in proteins; contains a secondary amino group 2) aromatic (R groups have phenyl ring) phenylalanine - very hydrophobic tyrosine - hydrophobic, but not as much because of polar groups tryptop ...
Bioenergetics and Metabolism
... intermediate glyceraldehyde-3-P (GAP) which is then oxidized to produce NADH and 1,3bisphosphoglycerate. The next four reactions lead to the production of FOUR total ATP because each glucose molecule results in the production of TWO pyruvate. The net yield of ATP in glycolysis is therefore TWO ATP. ...
... intermediate glyceraldehyde-3-P (GAP) which is then oxidized to produce NADH and 1,3bisphosphoglycerate. The next four reactions lead to the production of FOUR total ATP because each glucose molecule results in the production of TWO pyruvate. The net yield of ATP in glycolysis is therefore TWO ATP. ...
Document
... starch. Amylase is present in our saliva and in secretions from the pancreas, and begins to act on the starch in our food while still in the mouth. Starch is a polymer made from many glucose molecules connected together (figure at right). It is used by many organisms as a way to store glucose for la ...
... starch. Amylase is present in our saliva and in secretions from the pancreas, and begins to act on the starch in our food while still in the mouth. Starch is a polymer made from many glucose molecules connected together (figure at right). It is used by many organisms as a way to store glucose for la ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.