Chapter 9: Cellular Respiration: Harvesting Chemical Energy Living
... -The explosion of a gasoline tank cannot drive a car very far -Cellular respiration does not oxidize glucose in a single explosive step that transfers all the H from the fuel to the oxygen at one time -Broken down gradually in a series of steps -Each one catalyzed by an enzyme -At key steps H is str ...
... -The explosion of a gasoline tank cannot drive a car very far -Cellular respiration does not oxidize glucose in a single explosive step that transfers all the H from the fuel to the oxygen at one time -Broken down gradually in a series of steps -Each one catalyzed by an enzyme -At key steps H is str ...
Emerging therapeutic roles for NAD+ metabolism in mitochondrial
... Nicotinamide adenine dinucleotide (NAD+) is a central metabolic cofactor in eukaryotic cells that plays a critical role in regulating cellular metabolism and energy homeostasis. NAD+ in its reduced form (i.e. NADH) serves as the primary electron donor in mitochondrial respiratory chain, which involv ...
... Nicotinamide adenine dinucleotide (NAD+) is a central metabolic cofactor in eukaryotic cells that plays a critical role in regulating cellular metabolism and energy homeostasis. NAD+ in its reduced form (i.e. NADH) serves as the primary electron donor in mitochondrial respiratory chain, which involv ...
Lecture 15
... - Glycolysis occurs in the cytosol and breaks glucose into two pyruvates - Krebs Cycle takes place within the mitochondrial matrix, and breaks a pyruvate into CO2 and produce some ATP and NADH. - Some steps of Glycolysis and Krebs Cycle are Redox in which dehydrogenase enzyme reduces NAD+ into NADH. ...
... - Glycolysis occurs in the cytosol and breaks glucose into two pyruvates - Krebs Cycle takes place within the mitochondrial matrix, and breaks a pyruvate into CO2 and produce some ATP and NADH. - Some steps of Glycolysis and Krebs Cycle are Redox in which dehydrogenase enzyme reduces NAD+ into NADH. ...
File - Wk 1-2
... products of the cycle and the role of the cycle in providing reducing equivalents for the electron transport chain. The citric acid cycle (Krebs cycle) occurs in the mitacholdria of the cell and occurs in the presence of oxygen (aerobic pathway). Pyruvic acid from glycolysis first needs to be conver ...
... products of the cycle and the role of the cycle in providing reducing equivalents for the electron transport chain. The citric acid cycle (Krebs cycle) occurs in the mitacholdria of the cell and occurs in the presence of oxygen (aerobic pathway). Pyruvic acid from glycolysis first needs to be conver ...
Allosteric Enzymes
... • Because Ser-195 and His-57 are required for activity, they must be close to each other in the active site • The folding of the Chymotrypsin backbone, positions the essential amino acids around the active-site pocket in anti parallel pleated-sheet ...
... • Because Ser-195 and His-57 are required for activity, they must be close to each other in the active site • The folding of the Chymotrypsin backbone, positions the essential amino acids around the active-site pocket in anti parallel pleated-sheet ...
Biochemistry
... Bis is of two parts; Bi =ثنائي, while s = “separated” (i.e. on different locations) Glycerald. 3-P converts into 2,3 bis PG or 2,3 BPG or 1,3 DPG and is present in most cells at low concentrations, but in the RBCs (erythrocytes) it is at high concentration (4 mM) which is equal to hemoglobin. I ...
... Bis is of two parts; Bi =ثنائي, while s = “separated” (i.e. on different locations) Glycerald. 3-P converts into 2,3 bis PG or 2,3 BPG or 1,3 DPG and is present in most cells at low concentrations, but in the RBCs (erythrocytes) it is at high concentration (4 mM) which is equal to hemoglobin. I ...
Enzymes, ATP and Bioenergetics
... Though ATP is the most common form of nucleoside triphosphate, it is not the only one. Nucleotides containing the bases guanine, cytosine, thymine and uracil can also take on extra phosphate groups to form high-energy compounds. Nucleoside triphosphates (NTPs) may contain either ribose or and are s ...
... Though ATP is the most common form of nucleoside triphosphate, it is not the only one. Nucleotides containing the bases guanine, cytosine, thymine and uracil can also take on extra phosphate groups to form high-energy compounds. Nucleoside triphosphates (NTPs) may contain either ribose or and are s ...
Nutrients are chemical substances in food that provide energy, form
... vitamins are divided into two principal groups, fat soluble and water soluble. Heat is a form of energy that can be measured as temperature and expressed in units called calories. A calorie is the amount of heat energy required to raise the temperature of one gram of water one degree celsius. Since ...
... vitamins are divided into two principal groups, fat soluble and water soluble. Heat is a form of energy that can be measured as temperature and expressed in units called calories. A calorie is the amount of heat energy required to raise the temperature of one gram of water one degree celsius. Since ...
Exam 3 - Chemistry Courses: About
... are not taken directly into the mitochondria, but reducing equivalents are transported in through the ______________________ shuttle. B. Complex II, also called ___________________ from the citric acid cycle, adds to the pool of _________________ but does not transport any protons. C. The ultimate e ...
... are not taken directly into the mitochondria, but reducing equivalents are transported in through the ______________________ shuttle. B. Complex II, also called ___________________ from the citric acid cycle, adds to the pool of _________________ but does not transport any protons. C. The ultimate e ...
HOW CELLS HARVEST ENERGY
... As e- is moved thru ETC, the energy in e- is used to actively pump protons across the inner membrane NRG from the e- is now stored in the proton gradient As the protons diffuse down their concentration gradient, ATP synthase uses the energy in the gradient to make 32ATP by chemiosmotic phosphorylati ...
... As e- is moved thru ETC, the energy in e- is used to actively pump protons across the inner membrane NRG from the e- is now stored in the proton gradient As the protons diffuse down their concentration gradient, ATP synthase uses the energy in the gradient to make 32ATP by chemiosmotic phosphorylati ...
Mitochondria
... bread is starch, a polysaccharide that is readily broken down by the digestive system into its component monosaccharide, glucose. The resulting glucose molecules can be oxidized to produce ATP (catabolism) or they can be bound together to make another polysaccharide, glycogen (anabolism). Glycogen i ...
... bread is starch, a polysaccharide that is readily broken down by the digestive system into its component monosaccharide, glucose. The resulting glucose molecules can be oxidized to produce ATP (catabolism) or they can be bound together to make another polysaccharide, glycogen (anabolism). Glycogen i ...
Chapter 9 – Cellular Respiration and Fermentation
... life comes from the sun. Energy flows into an ecosystem as sunlight and exists as heat (Figure 9.2). Cells harvest chemical energy stored in organic molecules and use it to generate ATP. There are three (3) key pathways of respiration: 1) glycolysis, 2) the citric acid cycle, and 3) oxidative phosph ...
... life comes from the sun. Energy flows into an ecosystem as sunlight and exists as heat (Figure 9.2). Cells harvest chemical energy stored in organic molecules and use it to generate ATP. There are three (3) key pathways of respiration: 1) glycolysis, 2) the citric acid cycle, and 3) oxidative phosph ...
CELLULAR RESPIRATION
... PYRUVATE (3C) molecules The 6C glucose is phosphorylated then split into 2 triose phosphate molecules (3C) which are then oxidised further to produce the pyruvate, some ATP and reduced NAD NAD can be reduced to NADH - it accepts H+ and transports ions around the cell - the hydrogen can be transferre ...
... PYRUVATE (3C) molecules The 6C glucose is phosphorylated then split into 2 triose phosphate molecules (3C) which are then oxidised further to produce the pyruvate, some ATP and reduced NAD NAD can be reduced to NADH - it accepts H+ and transports ions around the cell - the hydrogen can be transferre ...
Cell Biology
... o If oxygen available, pyruvate fed into TCA cycle where it generates some ATP and more NADH(H+) and FADH2 are used to generate ATP by oxidative phosphorylation and chemiosmotic coupling via ETS. Oxidized to carbon dioxide. o If there is no oxygen available or cannot be used another way to regenerat ...
... o If oxygen available, pyruvate fed into TCA cycle where it generates some ATP and more NADH(H+) and FADH2 are used to generate ATP by oxidative phosphorylation and chemiosmotic coupling via ETS. Oxidized to carbon dioxide. o If there is no oxygen available or cannot be used another way to regenerat ...
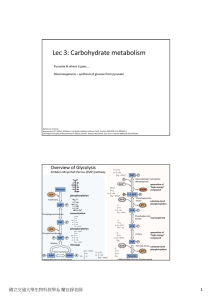
Lec 3: Carbohydrate metabolism
... Lots of heme‐containing mitochondria, used in aerobic metabolism ...
... Lots of heme‐containing mitochondria, used in aerobic metabolism ...
ENZYMES: THE MAJESTIC MOLECULES OF LIFE Part
... The primary, secondary, tertiary and quaternary structures of proteins are essential to their catalytic activity Enzymes are synthesized within the cell and they are operative at the site of their synthesis Enzymes change the rate or velocity of a biochemical reaction without being used up them ...
... The primary, secondary, tertiary and quaternary structures of proteins are essential to their catalytic activity Enzymes are synthesized within the cell and they are operative at the site of their synthesis Enzymes change the rate or velocity of a biochemical reaction without being used up them ...
Chapter 9 – Cellular Respiration and Fermentation
... life comes from the sun. Energy flows into an ecosystem as sunlight and exists as heat (Figure 9.2). Cells harvest chemical energy stored in organic molecules and use it to generate ATP. There are three (3) key pathways of respiration: 1) glycolysis, 2) the citric acid cycle, and 3) oxidative phosph ...
... life comes from the sun. Energy flows into an ecosystem as sunlight and exists as heat (Figure 9.2). Cells harvest chemical energy stored in organic molecules and use it to generate ATP. There are three (3) key pathways of respiration: 1) glycolysis, 2) the citric acid cycle, and 3) oxidative phosph ...
Slide 1
... Alkaptonuria is inherited, which means it is passed down from parents to their children. To get this disease, each of your parents must pass you a copy of the faulty HGD gene. Urine in an infant's diaper may darken and can turn almost black after several hours. However, many persons with this con ...
... Alkaptonuria is inherited, which means it is passed down from parents to their children. To get this disease, each of your parents must pass you a copy of the faulty HGD gene. Urine in an infant's diaper may darken and can turn almost black after several hours. However, many persons with this con ...
Ch. 6and7_Notes
... and the Krebs Cycle to produce reducing power in NADH and FADH -Describe where in the cell this takes place -Explain how chemiosmosis converts the reducing power of NADH and FADH to store chemical potential energy as ATP -Describe where in the mitochondrion this takes ...
... and the Krebs Cycle to produce reducing power in NADH and FADH -Describe where in the cell this takes place -Explain how chemiosmosis converts the reducing power of NADH and FADH to store chemical potential energy as ATP -Describe where in the mitochondrion this takes ...
video slide - Knappology
... • Dehydrogenase removes a pair of H atoms (2 e-, 2 p) from glucose thus oxidizing it. • Dehydrogenase delivers 2e- and 1 p to NAD+ and the other proton is releases as H+ ion. NAD+ is now NADH (stores energy for later use) ...
... • Dehydrogenase removes a pair of H atoms (2 e-, 2 p) from glucose thus oxidizing it. • Dehydrogenase delivers 2e- and 1 p to NAD+ and the other proton is releases as H+ ion. NAD+ is now NADH (stores energy for later use) ...
BIOCHEMISTRY
... can produce hundreds of ATPs (for example, the catabolism of a single 18-carbon fatty acid molecule produce 108 ATPs). Lipids are the most concentrated form of stored energy, containing by far the most calories per gram. ...
... can produce hundreds of ATPs (for example, the catabolism of a single 18-carbon fatty acid molecule produce 108 ATPs). Lipids are the most concentrated form of stored energy, containing by far the most calories per gram. ...
Sample Question Set 5a
... 3. Choose the best definition for a near-equilibrium reaction: (i) always operates with a favorable free energy change (ii) has a free energy change near zero (iii) is usually a control point in a metabolic pathway (iv) operates very slowly in vivo. 4. Creatine kinase catalyzes the following reactio ...
... 3. Choose the best definition for a near-equilibrium reaction: (i) always operates with a favorable free energy change (ii) has a free energy change near zero (iii) is usually a control point in a metabolic pathway (iv) operates very slowly in vivo. 4. Creatine kinase catalyzes the following reactio ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.