lecture1
... Metabolic pathways can be linear, e.g. glycolysis or can be cyclic, e.g. TCA. In general, the rate of catabolism is controlled not by the conc. of nutrients available in the environment of the cell, but by the cell’s need for energy in the form of ATP. Similarly, the rate of biosynthesis of cell com ...
... Metabolic pathways can be linear, e.g. glycolysis or can be cyclic, e.g. TCA. In general, the rate of catabolism is controlled not by the conc. of nutrients available in the environment of the cell, but by the cell’s need for energy in the form of ATP. Similarly, the rate of biosynthesis of cell com ...
Information Sheet
... Aerobic – in the presence of oxygen Anaerobic – in the absence of oxygen ATP (Adenosine triphosphate) – a molecule that stores energy in the form of phosphate bonds. The conversion of ATP ADP (Adenosine diphosphate) releases energy Fermentation – the chemical conversion of carbohydrates into alcoh ...
... Aerobic – in the presence of oxygen Anaerobic – in the absence of oxygen ATP (Adenosine triphosphate) – a molecule that stores energy in the form of phosphate bonds. The conversion of ATP ADP (Adenosine diphosphate) releases energy Fermentation – the chemical conversion of carbohydrates into alcoh ...
Khaled Hamarneh Summary
... Ring with 2 oxygen atoms “IN SEQUENTIAL MANNER” the ring’s function is to make an access for electrons ( because they must be donated with hydrocarbon tail ) .. the function of this tail is to pass freely through the membrane ( hydrophobic structure ) .. 1. Can accept 1 or 2 electrons, it may le ...
... Ring with 2 oxygen atoms “IN SEQUENTIAL MANNER” the ring’s function is to make an access for electrons ( because they must be donated with hydrocarbon tail ) .. the function of this tail is to pass freely through the membrane ( hydrophobic structure ) .. 1. Can accept 1 or 2 electrons, it may le ...
Class22 2-9 Win17 Respiration Regulation and
... How does the ATP yield of fermentation compared to that of aerobic respiration? In what conditions would the evolution of enzymes and regulation to allow fermentation be advantageous? ...
... How does the ATP yield of fermentation compared to that of aerobic respiration? In what conditions would the evolution of enzymes and regulation to allow fermentation be advantageous? ...
AP Biology Ch. 9 Cellular Respiration
... without oxygen. It only releases a small amount of ATP. Glycolysis: the first step of breaking down glucose—it splits glucose (6C) into 2 pyruvic acid molecules (3C each) ...
... without oxygen. It only releases a small amount of ATP. Glycolysis: the first step of breaking down glucose—it splits glucose (6C) into 2 pyruvic acid molecules (3C each) ...
Enzymes
... place in cells. 2. Enzymes are very specific, generally catalyzing only one chemical reaction. 3. For this reason, part of an enzyme’s name is usually derived from the reaction it catalyzes. Enzymes usually end in the suffix “–ase”. Ex. Alcohol dehydrogenase catalyzes the reaction that removes water ...
... place in cells. 2. Enzymes are very specific, generally catalyzing only one chemical reaction. 3. For this reason, part of an enzyme’s name is usually derived from the reaction it catalyzes. Enzymes usually end in the suffix “–ase”. Ex. Alcohol dehydrogenase catalyzes the reaction that removes water ...
Bio102 Problems
... AcCoA ATP, NADH and FADH2 Citric Acid Cycle Calvin-Benson Cycle ATP and NADPH Glucose (or Light-Independent Reactions) ...
... AcCoA ATP, NADH and FADH2 Citric Acid Cycle Calvin-Benson Cycle ATP and NADPH Glucose (or Light-Independent Reactions) ...
Principles of BIOCHEMISTRY
... • Lack of niacin causes the disease pellagra • Humans obtain niacin from cereals, meat, legumes ...
... • Lack of niacin causes the disease pellagra • Humans obtain niacin from cereals, meat, legumes ...
Chapter 7 7 The Behavior of Proteins: Enzymes Mechanisms and
... structurally related substrates to give structurally related l d products d • Stereospecificity: catalyzes a reaction in which one stereoisomer is reacted or formed in p preference to all others that might be reacted or formed ...
... structurally related substrates to give structurally related l d products d • Stereospecificity: catalyzes a reaction in which one stereoisomer is reacted or formed in p preference to all others that might be reacted or formed ...
CHAPTER 9: HOW CELLS HARVEST ENERGY
... out through specific transmembrane channels re-enter through other channels coupled to ATP synthesis. Most biological ATP is produced in this manner. Glycolysis occurs in the cytoplasm of a cell and is catalyzed by enzymes not associated with any membranes or organelles. Glucose is converted to two ...
... out through specific transmembrane channels re-enter through other channels coupled to ATP synthesis. Most biological ATP is produced in this manner. Glycolysis occurs in the cytoplasm of a cell and is catalyzed by enzymes not associated with any membranes or organelles. Glucose is converted to two ...
Nutrition and Metabolism (Chap 4)
... Breakdown of sugars to pyruvate and similar intermediates Some production of ATP (substrate-level phosphorylation) and reducing power (reduced coenzymes; NADH) Several pathways by which a cell can break down a sugar (sugars are the major substrates of catabolic energy releasing reactions used ...
... Breakdown of sugars to pyruvate and similar intermediates Some production of ATP (substrate-level phosphorylation) and reducing power (reduced coenzymes; NADH) Several pathways by which a cell can break down a sugar (sugars are the major substrates of catabolic energy releasing reactions used ...
Microbial Metabolism
... • Breakdown of sugars to pyruvate and similar intermediates • Some production of ATP (substrate-level phosphorylation) and reducing power (reduced coenzymes; NADH) • Several pathways by which a cell can break down a sugar (sugars are the major substrates of catabolic energy releasing reactions used ...
... • Breakdown of sugars to pyruvate and similar intermediates • Some production of ATP (substrate-level phosphorylation) and reducing power (reduced coenzymes; NADH) • Several pathways by which a cell can break down a sugar (sugars are the major substrates of catabolic energy releasing reactions used ...
Slide 1
... – In glycolysis, glucose is oxidized to 2 pyruvate molecules with NAD+ as the oxidizing agent (not O2). – Some energy from this oxidation produce 2 ATP. – If oxygen is present, additional ATP can be generated when NADH delivers its electrons to the electron transport chain. Glycolysis generates 2 AT ...
... – In glycolysis, glucose is oxidized to 2 pyruvate molecules with NAD+ as the oxidizing agent (not O2). – Some energy from this oxidation produce 2 ATP. – If oxygen is present, additional ATP can be generated when NADH delivers its electrons to the electron transport chain. Glycolysis generates 2 AT ...
Must-Knows: Unit 4 (Cellular Respiration) Ms. Ottolini, AP Biology
... matrix to the intermembrane space. As a result of this force, H+ “wants” to flow back down its gradient from a high concentration in the intermembrane space to a low concentration in the matrix. The only way that it can flow through the inner membrane is by passing through the ATP synthase protein. ...
... matrix to the intermembrane space. As a result of this force, H+ “wants” to flow back down its gradient from a high concentration in the intermembrane space to a low concentration in the matrix. The only way that it can flow through the inner membrane is by passing through the ATP synthase protein. ...
Metabolism
... How to Calculate Ideal Body Weight Males: 106 lb for 1st 5’ of ht, then 6 lb for each in over 5’ Females: 100 lb for 1st 5’ of ht, then 5 lb for each in over 5’ Add 10% for a large frame Subtract 10% for a small frame ...
... How to Calculate Ideal Body Weight Males: 106 lb for 1st 5’ of ht, then 6 lb for each in over 5’ Females: 100 lb for 1st 5’ of ht, then 5 lb for each in over 5’ Add 10% for a large frame Subtract 10% for a small frame ...
Cellular Respiration Releases Energy from Organic Compounds
... 2 intermediate 3-C molecules are formed 4 molecules of ATP are synthesized, and NAD+ is reduced to NADH 2 molecules of pyruvate are formed NET GAIN: 2 ATP and 2 pyruvate molecules ...
... 2 intermediate 3-C molecules are formed 4 molecules of ATP are synthesized, and NAD+ is reduced to NADH 2 molecules of pyruvate are formed NET GAIN: 2 ATP and 2 pyruvate molecules ...
BIS103-002 (Spring 2008) - UC Davis Plant Sciences
... combination of the reactions catalyzed by α-ketoglutarate dehydrogenase and succinyl-CoA synthetase). Initially, the energy released during the oxidation step is captured to form a thioester (covalently linked to glyceraldehyde-3-P dehydrogenase in glycolysis; succinyl-CoA in the TCA cycle). This th ...
... combination of the reactions catalyzed by α-ketoglutarate dehydrogenase and succinyl-CoA synthetase). Initially, the energy released during the oxidation step is captured to form a thioester (covalently linked to glyceraldehyde-3-P dehydrogenase in glycolysis; succinyl-CoA in the TCA cycle). This th ...
Electron Transport Chain - Dr-Manar-KSU
... shape of the molecule to work. When Transport e.g. membrane protein the phosphate group leaves the molecule, the molecule returns to its alternate shape. Chemical e.g. forming products from reactants. • An animal cell regenerates ATP from ADP Mechanical e.g. motor protein and Pi by the catabolism of ...
... shape of the molecule to work. When Transport e.g. membrane protein the phosphate group leaves the molecule, the molecule returns to its alternate shape. Chemical e.g. forming products from reactants. • An animal cell regenerates ATP from ADP Mechanical e.g. motor protein and Pi by the catabolism of ...
3.7 Cell Respiration
... pass from NADH/FADH2 to the final electron acceptor oxygen, which then combines with H+ and leaves as water. It also pumps H+ ions from the matrix to the intermembrane space. Chemiosmosis is the coupling of the electron transport chain and ATP synthase to move H+ ions from the matrix to the intermem ...
... pass from NADH/FADH2 to the final electron acceptor oxygen, which then combines with H+ and leaves as water. It also pumps H+ ions from the matrix to the intermembrane space. Chemiosmosis is the coupling of the electron transport chain and ATP synthase to move H+ ions from the matrix to the intermem ...
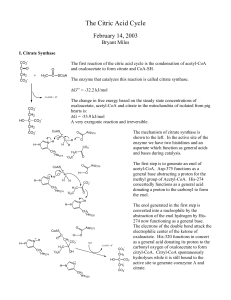
The Citric Acid Cycle
... energy change for hydrolyzing succinyl-CoA into succinate and coenzyme A is -33.8 kJ/mole. The standard free energy required to synthesize GTP from GDP and Pi is +30.5 kJ/mole. If we couple these two reactions together than the standard free energy change is -3.3 kJ/mole. This enzyme catalyzes a sub ...
... energy change for hydrolyzing succinyl-CoA into succinate and coenzyme A is -33.8 kJ/mole. The standard free energy required to synthesize GTP from GDP and Pi is +30.5 kJ/mole. If we couple these two reactions together than the standard free energy change is -3.3 kJ/mole. This enzyme catalyzes a sub ...
Metabolism
... • BMR is a measure of the minimum amount of energy required for body maintenance, partially related to amount of thyroxine produced (thyroid gland) which regulates rate of ATP use and is not altered by rest • Measured as kilocalories/kg body weight • Indirectly measured by oxygen consumption Energy ...
... • BMR is a measure of the minimum amount of energy required for body maintenance, partially related to amount of thyroxine produced (thyroid gland) which regulates rate of ATP use and is not altered by rest • Measured as kilocalories/kg body weight • Indirectly measured by oxygen consumption Energy ...
cell respiration
... energy found in NADH and FADH2 to make more ATP. This involves the cristae. There are electron transport chains that are used. The electrons from the NADH and FADH2 are used to move on the electron transport chain. As the electrons move down the electron transport chain, H+ ions are pumped across th ...
... energy found in NADH and FADH2 to make more ATP. This involves the cristae. There are electron transport chains that are used. The electrons from the NADH and FADH2 are used to move on the electron transport chain. As the electrons move down the electron transport chain, H+ ions are pumped across th ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.