Materiality: Is It Real?
... “measurement”, or observation. Particles are what comprise the atoms that we consider the building blocks of our material world. If particles are not substantial entities existing in space and enduring through time … what does that say about the atoms made from them that comprise our material reali ...
... “measurement”, or observation. Particles are what comprise the atoms that we consider the building blocks of our material world. If particles are not substantial entities existing in space and enduring through time … what does that say about the atoms made from them that comprise our material reali ...
3.3 Why do atoms radiate light?
... i.e. the dipole moment of the atom will change periodically. Consequently the atom serves as a sender for electromagnetic radiation with the frequency (ω2 − ω1 ). It will loose power in the state with the higher energy. After a short time the atom will be in the ground state which is not a mixed sta ...
... i.e. the dipole moment of the atom will change periodically. Consequently the atom serves as a sender for electromagnetic radiation with the frequency (ω2 − ω1 ). It will loose power in the state with the higher energy. After a short time the atom will be in the ground state which is not a mixed sta ...
Atomic Structure
... • All atoms of a given element are identical. • The atoms of a given element are different from those of any other element. John Dalton (1814) • Atoms of one element can combine with atoms of other elements to form compounds. A given compound always has the same relative numbers of types of atoms. ...
... • All atoms of a given element are identical. • The atoms of a given element are different from those of any other element. John Dalton (1814) • Atoms of one element can combine with atoms of other elements to form compounds. A given compound always has the same relative numbers of types of atoms. ...
form revision a
... Check your key area statements. If not green you need to do more work! Knowledge of the structure of the periodic table, groups and periods. All matter is made of atoms. When a substance contains only one kind of atom it is known as an element. Atoms contain protons, neutrons and electrons each with ...
... Check your key area statements. If not green you need to do more work! Knowledge of the structure of the periodic table, groups and periods. All matter is made of atoms. When a substance contains only one kind of atom it is known as an element. Atoms contain protons, neutrons and electrons each with ...
Inside the Atom connections to the lower secondary (KS3
... Pupils are taught about: • a simple (Dalton) atomic model • differences between atoms, elements and compounds • chemical symbols and formulae for elements and compounds • conservation of mass changes of state and chemical reactions. Most of the nuclear physics related content in the KS3 curricul ...
... Pupils are taught about: • a simple (Dalton) atomic model • differences between atoms, elements and compounds • chemical symbols and formulae for elements and compounds • conservation of mass changes of state and chemical reactions. Most of the nuclear physics related content in the KS3 curricul ...
Study Guide 1st Semester
... 37. What is the electron configuration for the following elements? (short hand or long hand is fine)? a) Gallium d) Magnesium b) Zirconium e) Iodine c) Californium f) Copper 38. What did Dalton, Thomson, Rutherford, Bohr, and Schrodinger have to say about the structure of the atoms (description and ...
... 37. What is the electron configuration for the following elements? (short hand or long hand is fine)? a) Gallium d) Magnesium b) Zirconium e) Iodine c) Californium f) Copper 38. What did Dalton, Thomson, Rutherford, Bohr, and Schrodinger have to say about the structure of the atoms (description and ...
The world of Atoms - University of California, Irvine
... “I cannot but confess that I attach only a transitory importance to this interpretation. I still believe in the possibility of a model of reality - that is to say, of a theory which represents things themselves and not merely the probability of their occurrence. On the other hand, it seems to me cer ...
... “I cannot but confess that I attach only a transitory importance to this interpretation. I still believe in the possibility of a model of reality - that is to say, of a theory which represents things themselves and not merely the probability of their occurrence. On the other hand, it seems to me cer ...
Chemistry 2100 In-Class Test 1(A)
... 6 pages of questions, and a formula/periodic table sheet (7 pages total). 2) If your work is not legible, it will be given a mark of zero. 3) Marks will be deducted for improper use of significant figures and for missing or incorrect units. 4) Show your work for all calculations. Answers without sup ...
... 6 pages of questions, and a formula/periodic table sheet (7 pages total). 2) If your work is not legible, it will be given a mark of zero. 3) Marks will be deducted for improper use of significant figures and for missing or incorrect units. 4) Show your work for all calculations. Answers without sup ...
Notes - Organization of Matter
... • Compounds are pure substances that are composed of two or more atoms that are chemically combined • Compounds can only be changed into simpler substances called elements by chemical changes ...
... • Compounds are pure substances that are composed of two or more atoms that are chemically combined • Compounds can only be changed into simpler substances called elements by chemical changes ...
PHY215: Study Guide for Introductory Quantum Mechanics Explain 1. Cathode Ray tubes, Cathode rays, and the generation of X‐rays.
... 1. Cathode Ray tubes, Cathode rays, and the generation of X‐rays. 2. The photoelectric effect, Compton Scattering, Planck’s constant: explain how light behaves as though it is made of particles. 3. The de Broglie wavelength, the Davisson‐Germer experiment: explain how electrons (an ...
... 1. Cathode Ray tubes, Cathode rays, and the generation of X‐rays. 2. The photoelectric effect, Compton Scattering, Planck’s constant: explain how light behaves as though it is made of particles. 3. The de Broglie wavelength, the Davisson‐Germer experiment: explain how electrons (an ...
Solid - burgess
... 2. Chemical changes can be accompanied by physical changes. 3. Identification of a chemical change involve careful examination of the original reactants to see if new substances (products) are produced. i. a gas is formed ii. temperature change iii. precipitate forms iv. appearance of a new color or ...
... 2. Chemical changes can be accompanied by physical changes. 3. Identification of a chemical change involve careful examination of the original reactants to see if new substances (products) are produced. i. a gas is formed ii. temperature change iii. precipitate forms iv. appearance of a new color or ...
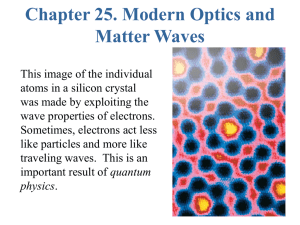
Chapter 31 Atomic Physics
... **** The idea that all matter is composed of atoms is fundamental to our modern view of the world. It has given us a firm basis for understanding the properties of solid, liquids, and gases. This understanding has led to a host of useful devices, one of the most famous being the laser. The laser be ...
... **** The idea that all matter is composed of atoms is fundamental to our modern view of the world. It has given us a firm basis for understanding the properties of solid, liquids, and gases. This understanding has led to a host of useful devices, one of the most famous being the laser. The laser be ...
NIELS BOHR power point22222
... This description of atomic structure is known as the Bohr atomic model. ...
... This description of atomic structure is known as the Bohr atomic model. ...
Section 5-2
... • Smaller orbit = lower energy level • Assigned the allowable electron orbitals the principle quantum number, n. • 1st orbit= lowest energy: n=1 • 2nd orbit= 2nd lowest energy: n=2 ...
... • Smaller orbit = lower energy level • Assigned the allowable electron orbitals the principle quantum number, n. • 1st orbit= lowest energy: n=1 • 2nd orbit= 2nd lowest energy: n=2 ...
41 Chapter 4 Atomic Structure 4.1 The Nuclear Atom J. J. Thomson
... bending them in magnetic fields. This let him find their charge/mass ratio. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. ("Raisin pudding.") This model may seem silly now, but, as we will see soon, some of the later, more sophisti ...
... bending them in magnetic fields. This let him find their charge/mass ratio. Thomson suggested (1898) that atoms consist of positively charged lumps of matter with electrons embedded in them. ("Raisin pudding.") This model may seem silly now, but, as we will see soon, some of the later, more sophisti ...
The Development of a New Atomic Model
... This explained the photoelectric effect • Einstein explained – Electromagnetic radiation is only absorbed by matter in whole numbers – In order for an electron to be rejected from the surface of the metal it must be struck by a single photon possessing at least a minimum energy required to knock th ...
... This explained the photoelectric effect • Einstein explained – Electromagnetic radiation is only absorbed by matter in whole numbers – In order for an electron to be rejected from the surface of the metal it must be struck by a single photon possessing at least a minimum energy required to knock th ...
Modern Physics 3-Atomic Physics
... The color of dyes results from the preferential absorption of certain wavelengths of light. For certain dyes, such as the cyanine dye of which a portion is illustrated below, we can determine the absorption wavelengths by using a simple particle in a box model. The dye molecules consist of symmetric ...
... The color of dyes results from the preferential absorption of certain wavelengths of light. For certain dyes, such as the cyanine dye of which a portion is illustrated below, we can determine the absorption wavelengths by using a simple particle in a box model. The dye molecules consist of symmetric ...
Physics 30 Atomic Model Review
... A Millikan wannabe set up an experiment using 2 parallel plates 6.0 cm apart with a potential difference of 1500 V. The mass of the hovering oil drop is calculated to be 1.63 x 10 -12g. a. Calculate the electric field strength. (2) ...
... A Millikan wannabe set up an experiment using 2 parallel plates 6.0 cm apart with a potential difference of 1500 V. The mass of the hovering oil drop is calculated to be 1.63 x 10 -12g. a. Calculate the electric field strength. (2) ...
Chemistry
... 95. A 78.0-g sample of an unknown compound contains 12.4 g of hydrogen. What is the percent by mass of hydrogen in the compound? ...
... 95. A 78.0-g sample of an unknown compound contains 12.4 g of hydrogen. What is the percent by mass of hydrogen in the compound? ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.