Shapes of the Charge Clouds
... •These areas are cloud-like with a fairly large space being filled by a few tiny electrons (e.g. fan blades) •Therefore, the electron cloud takes up most of the space of the atom, while the small, dense nucleus fills the center. ...
... •These areas are cloud-like with a fairly large space being filled by a few tiny electrons (e.g. fan blades) •Therefore, the electron cloud takes up most of the space of the atom, while the small, dense nucleus fills the center. ...
final exam practice test - Clayton State University
... 14. After interpreting the results of his oil drop experiment in 1909, Mullikan was able to determine____________________. a.) b.) c.) d.) e.) ...
... 14. After interpreting the results of his oil drop experiment in 1909, Mullikan was able to determine____________________. a.) b.) c.) d.) e.) ...
L37 - University of Iowa Physics
... • the neutrons and protons have about the same mass, and are each about 2000 times more massive than the electrons • the nucleus accounts for about 99.9% of the total mass of the atom • the neutrons have no charge what role do they play???? ...
... • the neutrons and protons have about the same mass, and are each about 2000 times more massive than the electrons • the nucleus accounts for about 99.9% of the total mass of the atom • the neutrons have no charge what role do they play???? ...
The role of atomic radius in ion channel selectivity :
... (total electrons for full shells). The result is the number of bonding electrons. 5. Assign 2 bonding electrons to each bond. 6. If bonding electrons remain, make some double or triple bonds. In general, double bonds form only between C, N, O, and S. Triple bonds are usually restricted to C, N, an ...
... (total electrons for full shells). The result is the number of bonding electrons. 5. Assign 2 bonding electrons to each bond. 6. If bonding electrons remain, make some double or triple bonds. In general, double bonds form only between C, N, O, and S. Triple bonds are usually restricted to C, N, an ...
Unit 4 Study Guide - Key - Effingham County Schools
... electrons from metals that have absorbed photons. 11. In terms of energy, what must happen for an atom to change from the ground state to an excited state? _absorb energy________________________ 12. If an electron is at its lowest energy it is in the _ground_____________ state. 13. What is the Heise ...
... electrons from metals that have absorbed photons. 11. In terms of energy, what must happen for an atom to change from the ground state to an excited state? _absorb energy________________________ 12. If an electron is at its lowest energy it is in the _ground_____________ state. 13. What is the Heise ...
1.1 What has to be explained by Quantum mechanics?
... Only reasonable for Fermions following the Pauli principle! But ”free” and ”occupied” states within a band, sizes of band gaps, etc. classify metals, semiconductors, and insulators. • Why, in contrast, must photons be Bosons?!? (One single QM state macroscopically measurable) • What is: Schrödinger ...
... Only reasonable for Fermions following the Pauli principle! But ”free” and ”occupied” states within a band, sizes of band gaps, etc. classify metals, semiconductors, and insulators. • Why, in contrast, must photons be Bosons?!? (One single QM state macroscopically measurable) • What is: Schrödinger ...
Unit 3 - Princeton High School
... The half-life for this disintegration is approximately 30 years. This is the amount of time required for half the atoms in a sample to undergo decay. Assume that a 64-gram sample of Cs-137 is analyzed every 30 years for a 150 –year period. Calculate the grams of cesium and barium present each time t ...
... The half-life for this disintegration is approximately 30 years. This is the amount of time required for half the atoms in a sample to undergo decay. Assume that a 64-gram sample of Cs-137 is analyzed every 30 years for a 150 –year period. Calculate the grams of cesium and barium present each time t ...
File - Flipped Out Science with Mrs. Thomas!
... law of conservation of mass 5E investigate how evidence of chemical reactions indicate that new substances with different properties are ...
... law of conservation of mass 5E investigate how evidence of chemical reactions indicate that new substances with different properties are ...
File - Flipped Out Science with Mrs. Thomas!
... law of conservation of mass 5E investigate how evidence of chemical reactions indicate that new substances with different properties are ...
... law of conservation of mass 5E investigate how evidence of chemical reactions indicate that new substances with different properties are ...
$doc.title
... with one oxygen atom to form one molecule of water. On the atomic scale, we never see an example of one and a half hydrogen atoms combining with an oxygen atom. This was one of the first observations of the early chemists who explored the properties of chemical elements. This observation is known as ...
... with one oxygen atom to form one molecule of water. On the atomic scale, we never see an example of one and a half hydrogen atoms combining with an oxygen atom. This was one of the first observations of the early chemists who explored the properties of chemical elements. This observation is known as ...
Summary - Physics
... make about the light detected by the spectrometer in experiment mode that Rutherford’s solar system model is unable to explain? If you look at light emitted by an atom through a spectrometer, you will see that only certain discrete colors are emitted. The classical solar system model does not predic ...
... make about the light detected by the spectrometer in experiment mode that Rutherford’s solar system model is unable to explain? If you look at light emitted by an atom through a spectrometer, you will see that only certain discrete colors are emitted. The classical solar system model does not predic ...
Chapter 28 Atomic Physics Wave Function, ψ The Heisenberg
... Question: The quantum theory of the atom (a) is based on the Bohr theory (b) is more comprehensive but less accurate than Bohr theory (c) cannot be reconciled with Newton’s laws of motion (d) is not based on a mechanical model and considers only observable quantities Answer: d ...
... Question: The quantum theory of the atom (a) is based on the Bohr theory (b) is more comprehensive but less accurate than Bohr theory (c) cannot be reconciled with Newton’s laws of motion (d) is not based on a mechanical model and considers only observable quantities Answer: d ...
Chapter 5 PPT/Notes A
... •Light is a type of electromagnetic radiation. •Light is a particle that travels as a wave. •See p 137 for parts of a wave. ...
... •Light is a type of electromagnetic radiation. •Light is a particle that travels as a wave. •See p 137 for parts of a wave. ...
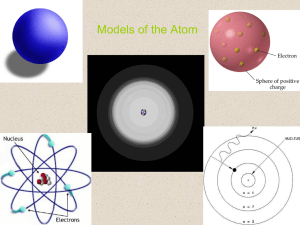
Models of the Atom
... Development of the Atomic Model • Could Rutherford’s atomic model explain the chemical properties of an element? No, to describe the chemical properties of an element we needed a model that better describes the behavior of electrons. ...
... Development of the Atomic Model • Could Rutherford’s atomic model explain the chemical properties of an element? No, to describe the chemical properties of an element we needed a model that better describes the behavior of electrons. ...
4.4 The Bohr Atom
... Again, it would be a good idea to read this section for ideas, but I won't test you on it. The main idea is that electrons and nuclei orbit each other. The much more massive nucleus moves very little, just as the earth does most of the orbiting around the sun. However, on the atomic scale, the corre ...
... Again, it would be a good idea to read this section for ideas, but I won't test you on it. The main idea is that electrons and nuclei orbit each other. The much more massive nucleus moves very little, just as the earth does most of the orbiting around the sun. However, on the atomic scale, the corre ...
Homework Set 1
... c. Taking λ/r ≤ 0.1 as the (arbitrary) cut-off when classical mechanics begins to be valid as Bohr’s quantum number n increases, calculate the lowest (smallest n) classical Bohr orbit. d. Using the Bohr theory, calculate the ionization energies in electron volts (eV) of hydrogen (H → H+ ) and of sin ...
... c. Taking λ/r ≤ 0.1 as the (arbitrary) cut-off when classical mechanics begins to be valid as Bohr’s quantum number n increases, calculate the lowest (smallest n) classical Bohr orbit. d. Using the Bohr theory, calculate the ionization energies in electron volts (eV) of hydrogen (H → H+ ) and of sin ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.