Electrons in Atoms 5.1 Worksheet
... This volume of space is described as an electron cloud, which has no boundary. The electron cloud is denser where the probability of finding the electron is high. Atomic Orbitals An atomic orbital describes where an electron is likely to be found. Numbered outward from the nucleus, each energy l ...
... This volume of space is described as an electron cloud, which has no boundary. The electron cloud is denser where the probability of finding the electron is high. Atomic Orbitals An atomic orbital describes where an electron is likely to be found. Numbered outward from the nucleus, each energy l ...
H 2 and H 2 + O 2 g H 2 O and H 2 O Hydrogen + Oxygen g Water
... Calcium + Chlorine → _________ ...
... Calcium + Chlorine → _________ ...
... These particles constituting the cathode rays were later called electrons. Since it was observed that the nature of cathode rays was the same irrespective of the metal used for the cathode or the gas filled in the cathode ray tube. This led Thomson to conclude that all atoms must contain electrons. ...
Periodic Table
... strong magnetic field are split. • Zeeman effect • Magnetic moment of orbiting electron Orbital magnetic number ml ...
... strong magnetic field are split. • Zeeman effect • Magnetic moment of orbiting electron Orbital magnetic number ml ...
Static Electricity Notes
... Let’s Start from the Beginning Atoms- smallest particle that makes up all matter. Electrons-Negative charged particles that make up an ...
... Let’s Start from the Beginning Atoms- smallest particle that makes up all matter. Electrons-Negative charged particles that make up an ...
Recitation 3
... Problem 20. In 1911, Ernest Rutherford and his assistants Hans Geiger and Ernest Mardsen conducted an experiment in which they scattered alpha particles from thin sheets of gold. An alpha particle, having a charge of qα = +2e and a mass of m = 6.64 · 10−27 kg is a product of certain radioactive deca ...
... Problem 20. In 1911, Ernest Rutherford and his assistants Hans Geiger and Ernest Mardsen conducted an experiment in which they scattered alpha particles from thin sheets of gold. An alpha particle, having a charge of qα = +2e and a mass of m = 6.64 · 10−27 kg is a product of certain radioactive deca ...
Document
... To create a beam of electrons for a double slit experiment, the electrons must have the same wavelength To have the same wavelength, the electrons must have the same momentum or equivalently, energy ...
... To create a beam of electrons for a double slit experiment, the electrons must have the same wavelength To have the same wavelength, the electrons must have the same momentum or equivalently, energy ...
Lecture
... Heat capacity of monoatomic solid Dulong and Petit’s law (valid for any monoatomic solid at high temp.) ...
... Heat capacity of monoatomic solid Dulong and Petit’s law (valid for any monoatomic solid at high temp.) ...
SEPARATION OF MATTER - Los Angeles City College
... • (Pure) substance: a material which can not be separated by physical methods into 2 or more materials which have different characteristics. • Compounds: a material containing two or more elements or molecules. • Molecules: the smallest grouping which a substance can be divided into without forming ...
... • (Pure) substance: a material which can not be separated by physical methods into 2 or more materials which have different characteristics. • Compounds: a material containing two or more elements or molecules. • Molecules: the smallest grouping which a substance can be divided into without forming ...
Analysis of a Matter
... • (Pure) substance: a material which can not be separated by physical methods into 2 or more materials which have different characteristics. • Compounds: a material containing two or more elements or molecules. • Molecules: the smallest grouping which a substance can be divided into without forming ...
... • (Pure) substance: a material which can not be separated by physical methods into 2 or more materials which have different characteristics. • Compounds: a material containing two or more elements or molecules. • Molecules: the smallest grouping which a substance can be divided into without forming ...
THE ATOM Elements Isotopes Ions
... If an atom was the same size as the school oval, the nucleus would be the size of a green pea on the cricket pitch and the electrons would be pinpoints moving around the boundary at rapid speeds. ...
... If an atom was the same size as the school oval, the nucleus would be the size of a green pea on the cricket pitch and the electrons would be pinpoints moving around the boundary at rapid speeds. ...
An Introduction To Particle Accelerators
... The Van de Graaff Generator This shows Robert Van de Graaff’s original high voltage generator at MIT in 1933 ...
... The Van de Graaff Generator This shows Robert Van de Graaff’s original high voltage generator at MIT in 1933 ...
WBL6_Lecture_Ch27
... The Bohr Theory of the Hydrogen Atom Atoms are observed to emit and absorb photons of particular wavelengths; each type of atom has its own set of wavelengths. An empirical formula was found that gives the four visible spectral lines of hydrogen: ...
... The Bohr Theory of the Hydrogen Atom Atoms are observed to emit and absorb photons of particular wavelengths; each type of atom has its own set of wavelengths. An empirical formula was found that gives the four visible spectral lines of hydrogen: ...
ABCT1742
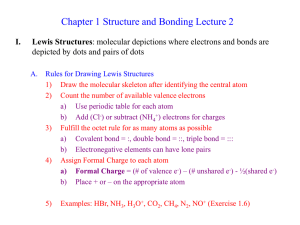
... Lewis theory and Octet rule, limitation of the Lewis theory, bond energies and bond distances, polar covalent bonds, VSEPR theory and molecular shapes of polyatomic molecules, physical properties and molecular shapes, Valence Bond theory Chemical Bonding – Delocalized Electron Pair Approach Princip ...
... Lewis theory and Octet rule, limitation of the Lewis theory, bond energies and bond distances, polar covalent bonds, VSEPR theory and molecular shapes of polyatomic molecules, physical properties and molecular shapes, Valence Bond theory Chemical Bonding – Delocalized Electron Pair Approach Princip ...
All of these can affect the rate at which a
... 8. Some types of weathering involve breaking up rock by agents such as roots or ice. What type of change is involved in this type of weathering? A a physical change B a chemical change C both a chemical change and a physical change D neither a chemical nor a physical change 9. Other types of weather ...
... 8. Some types of weathering involve breaking up rock by agents such as roots or ice. What type of change is involved in this type of weathering? A a physical change B a chemical change C both a chemical change and a physical change D neither a chemical nor a physical change 9. Other types of weather ...
Bohr`s atomic model: the evolution of a theory
... lines were found. Balmer checked them in his formula and these too (with m = 7, 8 etc) fitted. More spectral lines could be found by subtracting numbers larger than 22 . The generalization of Balmers formula reads 2 ...
... lines were found. Balmer checked them in his formula and these too (with m = 7, 8 etc) fitted. More spectral lines could be found by subtracting numbers larger than 22 . The generalization of Balmers formula reads 2 ...
Balmer Series
... Universe is made of hydrogen. Emission or absorption processes in hydrogen give rise to series, which are sequences of lines corresponding to atomic transitions, each ending or beginning with the same atomic state in hydrogen. Thus, for example, the Balmer Series involves transitions starting (for a ...
... Universe is made of hydrogen. Emission or absorption processes in hydrogen give rise to series, which are sequences of lines corresponding to atomic transitions, each ending or beginning with the same atomic state in hydrogen. Thus, for example, the Balmer Series involves transitions starting (for a ...
CH 6 electrons in atoms
... quantum number n increase. Thus, the spectral lines are also closer. Summarize de Broglie’s and Heisenberg’s contributions to the modern view of the atom. A serious problem with Bohr’s model is his assumption that the electron does not decay and lose energy as it moves in a circular path about the n ...
... quantum number n increase. Thus, the spectral lines are also closer. Summarize de Broglie’s and Heisenberg’s contributions to the modern view of the atom. A serious problem with Bohr’s model is his assumption that the electron does not decay and lose energy as it moves in a circular path about the n ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.