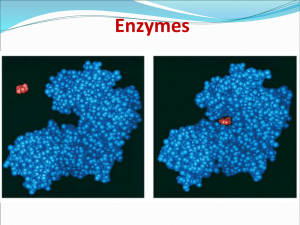
2nd CAT
... If we plot 1/v vs [I] at two different substrates concentration, we obtained the following graph: ...
... If we plot 1/v vs [I] at two different substrates concentration, we obtained the following graph: ...
Ch. 2-4 Review
... d. Excessive salt ions can cause an enzyme to denature. e. All of the above. 3. A cell uses energy released by ____ reactions to drive the ____ reaction that makes ATP. Then it uses the energy released by the hydrolysis of ATP, an ____ reaction, to do various kinds of work in the cell. a. exergonic… ...
... d. Excessive salt ions can cause an enzyme to denature. e. All of the above. 3. A cell uses energy released by ____ reactions to drive the ____ reaction that makes ATP. Then it uses the energy released by the hydrolysis of ATP, an ____ reaction, to do various kinds of work in the cell. a. exergonic… ...
Lecture #7
... activation energy required for a reaction to occur. The activation energy is reduced when the substrate attaches (by weak ionic attractions and hydrogen bonds) to the active site of the enzyme. The enzyme-substrate complex is known as the transition state. In the transition state, internal bonds of ...
... activation energy required for a reaction to occur. The activation energy is reduced when the substrate attaches (by weak ionic attractions and hydrogen bonds) to the active site of the enzyme. The enzyme-substrate complex is known as the transition state. In the transition state, internal bonds of ...
Bio301 Biochemistry I
... described by Michaelis-Menten kinetics. At a substrate concentration of 0.10 M, the initial rate of the reaction is 37 moles/min for a certain concentration of enzyme. However, you observe that at a lower substrate concentration of 0.010 M the initial reaction rate remains 37 moles/min. a) Using n ...
... described by Michaelis-Menten kinetics. At a substrate concentration of 0.10 M, the initial rate of the reaction is 37 moles/min for a certain concentration of enzyme. However, you observe that at a lower substrate concentration of 0.010 M the initial reaction rate remains 37 moles/min. a) Using n ...
J. Anim. Sci. Vol. 80, Suppl. 1/J. Dairy Sci. Vol. 85, Suppl. 1 414 Use
... random use of enzymes on feeds, without consideration for specific situations and substrate targets, will only discourage or delay on-farm adoption of enzyme technology. Research is needed to understand the mode of action of feed enzymes so that efficacy can be assured. While much progress has been ...
... random use of enzymes on feeds, without consideration for specific situations and substrate targets, will only discourage or delay on-farm adoption of enzyme technology. Research is needed to understand the mode of action of feed enzymes so that efficacy can be assured. While much progress has been ...
Enzymes
... enzyme helpers. They are components of coenzymes. Coenzymes make enzymes active by binding to the enzyme and making the active site the right shape for the substrate. Vitamin deficiency results in a decrease in enzymatic activity. Two major coenzymes are niacin (NAD) and flavin adenine dinucleotide ...
... enzyme helpers. They are components of coenzymes. Coenzymes make enzymes active by binding to the enzyme and making the active site the right shape for the substrate. Vitamin deficiency results in a decrease in enzymatic activity. Two major coenzymes are niacin (NAD) and flavin adenine dinucleotide ...
Diagnosis Test: EDEXCEL ADDITIONAL SCIENCE Biology
... 4. What are biological catalysts and state some reactions that they catalyse? ...
... 4. What are biological catalysts and state some reactions that they catalyse? ...
What Do Enzymes Do
... When there is enough product at the end of a reaction pathway (red macromolecule), it can inhibit its own synthesis by interacting with enzymes in the synthesis pathway (red arrow). © 2010 Nature Education All rights reserved. Figure Detail Not only do cells need to balance catabolic and anabolic pa ...
... When there is enough product at the end of a reaction pathway (red macromolecule), it can inhibit its own synthesis by interacting with enzymes in the synthesis pathway (red arrow). © 2010 Nature Education All rights reserved. Figure Detail Not only do cells need to balance catabolic and anabolic pa ...
CHM 112
... Lipids have large, non-polar hydrocarbon sections which are not attracted to water. Carbohydrates have multiple hydroxyl groups that form hydrogen bonds easily with water so the interactions, and thus the solubility, are greater. ...
... Lipids have large, non-polar hydrocarbon sections which are not attracted to water. Carbohydrates have multiple hydroxyl groups that form hydrogen bonds easily with water so the interactions, and thus the solubility, are greater. ...
Epjj Lecture 4
... bind to the enzyme, which results in the formation of the enzyme-substrate complex (ie E-S complex). The E-S complex forms rapidly in the first part of the enzyme catalysis process and the concentration of the E-S stays constant at a steady-state level. For this reason, this type of kinetics is ca ...
... bind to the enzyme, which results in the formation of the enzyme-substrate complex (ie E-S complex). The E-S complex forms rapidly in the first part of the enzyme catalysis process and the concentration of the E-S stays constant at a steady-state level. For this reason, this type of kinetics is ca ...
Executive Stress Formula
... occur during the course of normal metabolic processes. Many chemical reactions require significant energy in order to take place, and therefore need a catalyst to allow the reaction to proceed. The catalyst acts to lower the energy needed for the reaction to move forward. In the body, enzymes play t ...
... occur during the course of normal metabolic processes. Many chemical reactions require significant energy in order to take place, and therefore need a catalyst to allow the reaction to proceed. The catalyst acts to lower the energy needed for the reaction to move forward. In the body, enzymes play t ...
lab1
... Enzymes are proteins that catalyze (i.e., increase the rates of) chemical reactions Nearly all known enzymes are proteins in nature with the exception of certain RNA molecules can be effective biocatalysts too. These RNA molecules have come to be known as ribozymes. synthesized by the living cel ...
... Enzymes are proteins that catalyze (i.e., increase the rates of) chemical reactions Nearly all known enzymes are proteins in nature with the exception of certain RNA molecules can be effective biocatalysts too. These RNA molecules have come to be known as ribozymes. synthesized by the living cel ...
Chapter 6: An Introduction to Proteins
... --The serine has a polar hydroxyl, with the oxygen functioning as an electronegative nucleophile. A nearby histidine residue, with pKa » 6.0, however, can function as a base to abstract the proton from the serine hydroxyl group. The result of transfering the proton from the serine hydroxyl to the hi ...
... --The serine has a polar hydroxyl, with the oxygen functioning as an electronegative nucleophile. A nearby histidine residue, with pKa » 6.0, however, can function as a base to abstract the proton from the serine hydroxyl group. The result of transfering the proton from the serine hydroxyl to the hi ...
Enzyme Kinetics
... Importance of Enzyme Kinetics • Characteristic property and functions of E is the catalysis of chemical reaction. • Catalytic function can be studied by measurement of the rate of the catalysed reaction. • Is essential for detailed study of an E. • Helps define the best condition for the action of ...
... Importance of Enzyme Kinetics • Characteristic property and functions of E is the catalysis of chemical reaction. • Catalytic function can be studied by measurement of the rate of the catalysed reaction. • Is essential for detailed study of an E. • Helps define the best condition for the action of ...
Enzymes Notes - The Lesson Locker
... site is like a pocket into which the substrate fits. There is specificity between the enzyme and substrate because of the shape of the active site. i. As the substrate enters the active site, interactions between the substrate and the amino acids of the enzyme causes the enzyme to change shape sligh ...
... site is like a pocket into which the substrate fits. There is specificity between the enzyme and substrate because of the shape of the active site. i. As the substrate enters the active site, interactions between the substrate and the amino acids of the enzyme causes the enzyme to change shape sligh ...
File
... a role in its affinity to the enzyme d. Probability stays the same because the substrate and inhibitor have an equal degree of affinity to the enzyme 20. When an allosteric inhibitor binds to a protein, this will ________ the protein’s ability to bind to a substrate. a. Increase b. Decrease c. Catal ...
... a role in its affinity to the enzyme d. Probability stays the same because the substrate and inhibitor have an equal degree of affinity to the enzyme 20. When an allosteric inhibitor binds to a protein, this will ________ the protein’s ability to bind to a substrate. a. Increase b. Decrease c. Catal ...
File
... Although the lock and key model is an obvious staging post, ensure that students can distinguish between it and the induced fit model. It is useful to relate the structure of an enzyme and the specificity of the active site back to more general ideas about protein structure. The idea of activa ...
... Although the lock and key model is an obvious staging post, ensure that students can distinguish between it and the induced fit model. It is useful to relate the structure of an enzyme and the specificity of the active site back to more general ideas about protein structure. The idea of activa ...
Chapter 8 Enzyme PPT
... Competitive inhibitor: binds to the active site of an enzyme, competes with substrate Noncompetitive inhibitor: binds to another part of an enzyme enzyme changes shape active site is nonfunctional ...
... Competitive inhibitor: binds to the active site of an enzyme, competes with substrate Noncompetitive inhibitor: binds to another part of an enzyme enzyme changes shape active site is nonfunctional ...
enzymes - segaran1996
... Enzymes are proteins, so ..... Sensitive to temperature Sensitive to pH changes. ...
... Enzymes are proteins, so ..... Sensitive to temperature Sensitive to pH changes. ...
Chem 464 Biochemistry
... Biochemistry Multiple choice (4 points apiece): 1.In the binding of oxygen to myoglobin, the relationship between the concentration of oxygen and the fraction of binding sites occupied can best be described as: A) hyperbolic. B) linear with a negative slope. C) linear with a positive slope. D) rando ...
... Biochemistry Multiple choice (4 points apiece): 1.In the binding of oxygen to myoglobin, the relationship between the concentration of oxygen and the fraction of binding sites occupied can best be described as: A) hyperbolic. B) linear with a negative slope. C) linear with a positive slope. D) rando ...
Enzyme inhibitor

An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.The binding of an inhibitor can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. Inhibitor binding is either reversible or irreversible. Irreversible inhibitors usually react with the enzyme and change it chemically (e.g. via covalent bond formation). These inhibitors modify key amino acid residues needed for enzymatic activity. In contrast, reversible inhibitors bind non-covalently and different types of inhibition are produced depending on whether these inhibitors bind to the enzyme, the enzyme-substrate complex, or both.Many drug molecules are enzyme inhibitors, so their discovery and improvement is an active area of research in biochemistry and pharmacology. A medicinal enzyme inhibitor is often judged by its specificity (its lack of binding to other proteins) and its potency (its dissociation constant, which indicates the concentration needed to inhibit the enzyme). A high specificity and potency ensure that a drug will have few side effects and thus low toxicity.Enzyme inhibitors also occur naturally and are involved in the regulation of metabolism. For example, enzymes in a metabolic pathway can be inhibited by downstream products. This type of negative feedback slows the production line when products begin to build up and is an important way to maintain homeostasis in a cell. Other cellular enzyme inhibitors are proteins that specifically bind to and inhibit an enzyme target. This can help control enzymes that may be damaging to a cell, like proteases or nucleases. A well-characterised example of this is the ribonuclease inhibitor, which binds to ribonucleases in one of the tightest known protein–protein interactions. Natural enzyme inhibitors can also be poisons and are used as defences against predators or as ways of killing prey.