Enzymes
... The enzyme is thought to reduce the "path" of the reaction. This shortened path would require less energy for each molecule of substrate converted to product. Given a total amount of available energy, more molecules of substrate would be converted when the enzyme is present (the shortened "path") th ...
... The enzyme is thought to reduce the "path" of the reaction. This shortened path would require less energy for each molecule of substrate converted to product. Given a total amount of available energy, more molecules of substrate would be converted when the enzyme is present (the shortened "path") th ...
Unit 4 Test Review-Biomolecules Name Period ______ 1. Complete
... 24. Which is synthesized through the formation of peptide bonds? Protein 25. What are the differences between a saturated and unsaturated fat (min of 2)? What chemical structure accounts for this difference? Explain the effects of saturated versus unsaturated fats on a person’s health. Saturated fat ...
... 24. Which is synthesized through the formation of peptide bonds? Protein 25. What are the differences between a saturated and unsaturated fat (min of 2)? What chemical structure accounts for this difference? Explain the effects of saturated versus unsaturated fats on a person’s health. Saturated fat ...
active site - Blue Valley Schools
... site stabilizes favorable conformational changes at all Binding of one substrate molecule to other subunits. active site of one subunit locks all ...
... site stabilizes favorable conformational changes at all Binding of one substrate molecule to other subunits. active site of one subunit locks all ...
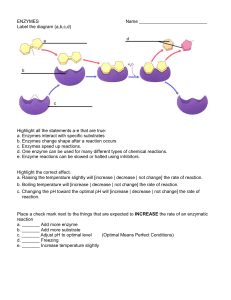
ENZYMES
... a. _______ Add more enzyme b. _______ Add more substrate c. _______ Adjust pH to optimal level (Optimal Means Perfect Conditions) d. _______ Freezing e. _______ Increase temperature slightly ...
... a. _______ Add more enzyme b. _______ Add more substrate c. _______ Adjust pH to optimal level (Optimal Means Perfect Conditions) d. _______ Freezing e. _______ Increase temperature slightly ...
Clicker game ?`s
... 13. Groups of photosynthetic pigments molecules situated in the thylakoid membrane are called A photosystems B carotenoids A chlorophyll B grana C proton pumps 14 In one type of enzyme regulation, the presence of the end product of a metabolic pathway inhibits an enzyme that catalyzes an early step ...
... 13. Groups of photosynthetic pigments molecules situated in the thylakoid membrane are called A photosystems B carotenoids A chlorophyll B grana C proton pumps 14 In one type of enzyme regulation, the presence of the end product of a metabolic pathway inhibits an enzyme that catalyzes an early step ...
Biochemistry I, Spring Term 2001 - Second Exam:
... ii) Briefly discuss transition state theory as it applies to the rate enhancement of enzymatic reactions. Provide one concrete example of how enzymes might affect the energy of the transition state. ...
... ii) Briefly discuss transition state theory as it applies to the rate enhancement of enzymatic reactions. Provide one concrete example of how enzymes might affect the energy of the transition state. ...
Enzymes are Most Effective at Optimal Conditions
... Under the influence of very high temperature, the weak H-bonds of the enzyme tend to break, due to which the rate of reaction decreases or stops all together. In other words, a denatured enzyme fails to carry out its normal functions. In the human body, the optimum temperature at which most enzymes ...
... Under the influence of very high temperature, the weak H-bonds of the enzyme tend to break, due to which the rate of reaction decreases or stops all together. In other words, a denatured enzyme fails to carry out its normal functions. In the human body, the optimum temperature at which most enzymes ...
Basic Enzyme Kinetics
... concentration of 1 unit (E and E S curves have been scale by two on the graph). In the central portion of the plots one can observe the relatively steady concentrations of E S and E (dE S/dt ≈ 0). At the same time, the rate of change of S and P are constant over this period. k1 = 100; k2 = 1; k3 = 1 ...
... concentration of 1 unit (E and E S curves have been scale by two on the graph). In the central portion of the plots one can observe the relatively steady concentrations of E S and E (dE S/dt ≈ 0). At the same time, the rate of change of S and P are constant over this period. k1 = 100; k2 = 1; k3 = 1 ...
October 12 AP Biology - John D. O`Bryant School of Math & Science
... E) weak interactions form between inhibitor and enzyme. ...
... E) weak interactions form between inhibitor and enzyme. ...
Enzymes - Mr. hawkins
... spherical or globular shape. The overall 3D shape of an enzyme molecule is very important: if it is altered, the enzyme cannot bind to its substrate and so cannot function. Enzyme shape is maintained by hydrogen bonds and ionic forces. Enzymes have several important properties: Enzymes are specifi ...
... spherical or globular shape. The overall 3D shape of an enzyme molecule is very important: if it is altered, the enzyme cannot bind to its substrate and so cannot function. Enzyme shape is maintained by hydrogen bonds and ionic forces. Enzymes have several important properties: Enzymes are specifi ...
Faculty of Science, IUG
... Date:2/12 /2005 Name----------- & NO.----------Answer the following I- Sketch the titration curve, calculate pI and determine the regions of the buffer capacity of ASP. The pK values of its Alfa COOH, Alfa amino , and Beta COOH groups are 2.1, 9.2, and 3.9 respectively. (6pts). ...
... Date:2/12 /2005 Name----------- & NO.----------Answer the following I- Sketch the titration curve, calculate pI and determine the regions of the buffer capacity of ASP. The pK values of its Alfa COOH, Alfa amino , and Beta COOH groups are 2.1, 9.2, and 3.9 respectively. (6pts). ...
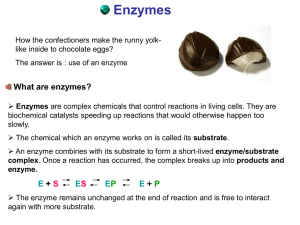
Enzyme Catalysis
... required to activate the reaction of the substrate molecule so that the products (P) of the reaction are formed. In summary: E + S ES E + P Note that the enzyme is not changed in the reaction and can even be recycled to break down additional substrate molecules. Each enzyme is specific for a par ...
... required to activate the reaction of the substrate molecule so that the products (P) of the reaction are formed. In summary: E + S ES E + P Note that the enzyme is not changed in the reaction and can even be recycled to break down additional substrate molecules. Each enzyme is specific for a par ...
Enzymes - Kevan Kruger
... Product ‘D’ can temporarily attach to an active site on Enzyme #1. The enzyme will be denatured and will not bind to reactant A. This is an example of NEGATIVE FEEDBACK or FEEDBACK INHIBITION, and it will stop the reaction. When the concentration of the product F gets low again, the inhibitor (F) w ...
... Product ‘D’ can temporarily attach to an active site on Enzyme #1. The enzyme will be denatured and will not bind to reactant A. This is an example of NEGATIVE FEEDBACK or FEEDBACK INHIBITION, and it will stop the reaction. When the concentration of the product F gets low again, the inhibitor (F) w ...
Document
... Graph (a) shows that this enzyme will work best at what type of temperatures? Graph (b) shows that this enzyme will work best at what type of temperatures? Graph (c) shows that this enzyme will work best at what type of pH? Graph (d) shows that this enzyme will work best at what type of pH? ...
... Graph (a) shows that this enzyme will work best at what type of temperatures? Graph (b) shows that this enzyme will work best at what type of temperatures? Graph (c) shows that this enzyme will work best at what type of pH? Graph (d) shows that this enzyme will work best at what type of pH? ...
Section: Energy and Chemical Reactions
... Energy is the ability to move or change matter. Activation energy is the energy needed to start a chemical reaction. DNA is a nucleic acid that stores hereditary information used to make proteins. RNA is a nucleic acid that is involved in protein synthesis. ATP is an organic molecule that acts as th ...
... Energy is the ability to move or change matter. Activation energy is the energy needed to start a chemical reaction. DNA is a nucleic acid that stores hereditary information used to make proteins. RNA is a nucleic acid that is involved in protein synthesis. ATP is an organic molecule that acts as th ...
Lecture 6 POWERPOINT here
... Increase the rate of virtually ALL chemical reactions - fact: A reaction that takes just milliseconds in the presence of an enzyme would take millions of years without (some increase the rate by as much as 1 x 1018 fold!!!) Enzyme pool selectively determines which reactions shall take place insi ...
... Increase the rate of virtually ALL chemical reactions - fact: A reaction that takes just milliseconds in the presence of an enzyme would take millions of years without (some increase the rate by as much as 1 x 1018 fold!!!) Enzyme pool selectively determines which reactions shall take place insi ...
enzyme
... designed to work best at pH 2 while those in the intestine are optimal at pH 8, both matching their working environments. ...
... designed to work best at pH 2 while those in the intestine are optimal at pH 8, both matching their working environments. ...
TABLE 3–1 Some Common Types of Enzymes
... break down nucleic acids by hydrolyzing bonds between nucleotides. break down proteins by hydrolyzing bonds between amino acids. general name used for enzymes that synthesize molecules in anabolic reactions by condensing two smaller molecules together. catalyze the rearrangement of bonds within a si ...
... break down nucleic acids by hydrolyzing bonds between nucleotides. break down proteins by hydrolyzing bonds between amino acids. general name used for enzymes that synthesize molecules in anabolic reactions by condensing two smaller molecules together. catalyze the rearrangement of bonds within a si ...
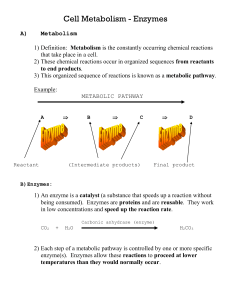
Chapter 4 - Enzymes and Energy
... • Many enzymes work by orienting molecules so that they can better contact each other. • Each type of enzyme has has a highly-ordered, characteristic 3-dimensional shape (conformation). • Ridges, grooves, and pockets lined with specific ...
... • Many enzymes work by orienting molecules so that they can better contact each other. • Each type of enzyme has has a highly-ordered, characteristic 3-dimensional shape (conformation). • Ridges, grooves, and pockets lined with specific ...
doc - University of California, Santa Cruz
... about the biological and evolutionary significance of introns. We therefore need a simple way of investigating those, and the enzymes involved in the intron turnover pathway. The target enzyme of the study, the RNA lariat debranching enzyme (DBR) from mosquito-borne parasitic protozoan Plasmodium fa ...
... about the biological and evolutionary significance of introns. We therefore need a simple way of investigating those, and the enzymes involved in the intron turnover pathway. The target enzyme of the study, the RNA lariat debranching enzyme (DBR) from mosquito-borne parasitic protozoan Plasmodium fa ...
honors Chapter 2.3-2.4 teaching
... – Competitive inhibitor: blocks active site, substrate can’t attach and remains unchanged – Non-competitive inhibitor: alters enzyme’s function by changing its shape • many poisons, pesticides, and drugs are enzyme inhibitors Some food for thought: 1. Why do we put lemon juice on apples? ...
... – Competitive inhibitor: blocks active site, substrate can’t attach and remains unchanged – Non-competitive inhibitor: alters enzyme’s function by changing its shape • many poisons, pesticides, and drugs are enzyme inhibitors Some food for thought: 1. Why do we put lemon juice on apples? ...
Enzyme inhibitor

An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.The binding of an inhibitor can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. Inhibitor binding is either reversible or irreversible. Irreversible inhibitors usually react with the enzyme and change it chemically (e.g. via covalent bond formation). These inhibitors modify key amino acid residues needed for enzymatic activity. In contrast, reversible inhibitors bind non-covalently and different types of inhibition are produced depending on whether these inhibitors bind to the enzyme, the enzyme-substrate complex, or both.Many drug molecules are enzyme inhibitors, so their discovery and improvement is an active area of research in biochemistry and pharmacology. A medicinal enzyme inhibitor is often judged by its specificity (its lack of binding to other proteins) and its potency (its dissociation constant, which indicates the concentration needed to inhibit the enzyme). A high specificity and potency ensure that a drug will have few side effects and thus low toxicity.Enzyme inhibitors also occur naturally and are involved in the regulation of metabolism. For example, enzymes in a metabolic pathway can be inhibited by downstream products. This type of negative feedback slows the production line when products begin to build up and is an important way to maintain homeostasis in a cell. Other cellular enzyme inhibitors are proteins that specifically bind to and inhibit an enzyme target. This can help control enzymes that may be damaging to a cell, like proteases or nucleases. A well-characterised example of this is the ribonuclease inhibitor, which binds to ribonucleases in one of the tightest known protein–protein interactions. Natural enzyme inhibitors can also be poisons and are used as defences against predators or as ways of killing prey.