* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download lab1
Restriction enzyme wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Inositol-trisphosphate 3-kinase wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Beta-lactamase wikipedia , lookup
Transferase wikipedia , lookup
Alcohol dehydrogenase wikipedia , lookup
Lactoylglutathione lyase wikipedia , lookup
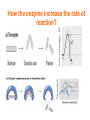
بسم هللا الرحمن الرحيم 221كيح علم اإلنزيمات أ /أروى الخيـــــــــــــــــــاط [email protected] مبنى 8الدور 3مكتب704 يوم الثالثاء 3-11 Introduction Enzymology Beginning story Louis Pasteur 19th century Wilhelm Kühne In 1878 Eduard Buchner In 1897 As early as the late 1700s and early 1800s, the digestion of meat by stomach secretions and the conversion of starch to sugars by plant extracts and saliva were known. However, the mechanism by which this occurred had not been identified In the 19th century, when studying the fermentation of sugar to alcohol by yeast, Louis Pasteur came to the conclusion that this fermentation was catalyzed by a vital force contained within the yeast cells called "ferments", which were thought to function only within living organisms. He wrote that "alcoholic fermentation is an act correlated with the life and organization of the yeast cells, not with the death or putrefaction of the cells. In 1878, German physiologist Wilhelm Kühne first used the term enzyme, which comes from Greek ενζυμον, "in leaven", to describe this process. The word enzyme was used later to refer to nonliving substances such as pepsin, and the word ferment was used to refer to chemical activity produced by living organisms. In 1897, Eduard Buchner began to study the ability of yeast extracts that lacked any living yeast cells to ferment sugar. In a series of experiments at the University of Berlin, he found that the sugar was fermented even when there were no living yeast cells in the mixture. He named the enzyme that brought about the fermentation of sucrose "zymase". In 1907, he received the Nobel Prize in Chemistry "for his biochemical research and his discovery of cell-free fermentation". Definition Catalyst are the substance that accelerate chemical reaction Organic catalyst (enzyme) 2 Mg,2 Cl-) Inorganic catalyst(Zn, Enzymes are proteins that catalyze (i.e., increase the rates of) chemical reactions Nearly all known enzymes are proteins in nature with the exception of certain RNA molecules can be effective biocatalysts too. These RNA molecules have come to be known as ribozymes. synthesized by the living cells In enzymatic reactions, the molecules at the beginning of the process are called substrates(S), and the enzyme converts them into different molecules, called the products(P) S E P+E In Biochemistry substrate is a molecule or substance upon which an enzyme acts. Products is a substance produced as a result of the reactions. Note: Enzyme Catalyze the chemical reactions without consumed Enzyme catalyzed reactions are mostly reversible HOW THE ENZYME BIND WITH THE SUBSTRATE? The enzyme can bind with the substrate by specific pocket on the enzyme known as active sites which define as small groove or area on the surface of the enzyme into which the substrate fits. that involve the formation of an intermediate enzyme-substrate complex S+E ES enzyme-substrate complex P+E How the active site become fits to substrate? Models that explain the enzyme specific towards substrate: Lock and key" model: Enzymes are very specific, because both the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. Induced fit model: The hypothesis suggested a modification to the lock and key model: since enzymes are rather flexible structures, the active site is continually reshaped by interactions with the substrate as the substrate interacts with the enzyme. As a result, the substrate does not simply bind to a rigid active site; the amino acid side chains which make up the active site are moulded into the precise positions that enable the enzyme to perform its catalytic function. How the enzyme increase the rate of reaction? The enzyme accelerate the rate of reaction through 1.Decreasing energy of activation without change free energy Activation energy ΔG: difference in the energy level of substrate or product and transition state (i.e. ΔG = rate of the reaction) How the enzyme increase the rate of reaction? Catalytic functional group of enzyme interact with functional group of S by weak bounds to form E S complex which causes release small amount of free energy, this energy that result from ES interaction called binding energy, which the enzyme used free energy to decrease activation energy Turnover number: Define as capable of combining enzyme with a given Total number of substrate molecules per minute. •This tells how many S molecules are converted to product by each enzyme molecule. •It tells us how fast an enzyme work or turnover S into P. •The turnover number varies from enzyme to enzyme. e.g. for catalase:turnover number is 5 x 106 for α-amylase→it is 1.9 x 104 This indicates that catalase is ~ 250 times more active than amylase