Enzymes
... • Active sites are highly specific and discriminatory i.e. sucrase does not accept lactose ...
... • Active sites are highly specific and discriminatory i.e. sucrase does not accept lactose ...
Arachidonic - University of Puget Sound
... •Rofecoxib (Vioxx) popular and effective, but withdrawn due to ...
... •Rofecoxib (Vioxx) popular and effective, but withdrawn due to ...
enzyme
... • T/F Carbon can stably bind with other carbons? • What is the monomer of a protein? • BONUS – What is dehydration synthesis? What is hydrolysis? ...
... • T/F Carbon can stably bind with other carbons? • What is the monomer of a protein? • BONUS – What is dehydration synthesis? What is hydrolysis? ...
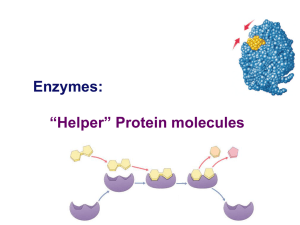
Enzymes: “Helper” Protein molecules
... sucrase breaks down sucrose Oh, I get it! They end in -ase ...
... sucrase breaks down sucrose Oh, I get it! They end in -ase ...
ENZYMES Characteristics of enzymes: Enzymes are proteins
... Names end in – ase ex. Maltase – this enzyme breaks down MALTOSE into two glucose molecules Lipase – breaks down lipids ...
... Names end in – ase ex. Maltase – this enzyme breaks down MALTOSE into two glucose molecules Lipase – breaks down lipids ...
Enzyme Catalysis Introduction
... near the active site to change its shape or block it. Many well known poisons such as potassium-cyanide and curare are enzyme inhibitors that interfere with the active site of critical enzymes. The enzyme used in this lab, catalase, has four polypeptide chains, each composed of more than 500 amino a ...
... near the active site to change its shape or block it. Many well known poisons such as potassium-cyanide and curare are enzyme inhibitors that interfere with the active site of critical enzymes. The enzyme used in this lab, catalase, has four polypeptide chains, each composed of more than 500 amino a ...
PBHS AP Biology
... illustrate the themes of this class. These labs are very important as the AP Test will have least one essay question and several multiple choice questions based on these labs. ...
... illustrate the themes of this class. These labs are very important as the AP Test will have least one essay question and several multiple choice questions based on these labs. ...
Enzyme Inhibition
... enzymic reactions The are usually specific and they work at low concentrations They block the enzyme but they do not usually destroy it Many drugs and poisons are inhibitors of enzymes in the nervous system ...
... enzymic reactions The are usually specific and they work at low concentrations They block the enzyme but they do not usually destroy it Many drugs and poisons are inhibitors of enzymes in the nervous system ...
Chemistry of Life - El Camino College
... A protein’s sequence of amino acids determines its shape which determines … ...
... A protein’s sequence of amino acids determines its shape which determines … ...
Study Guide
... 3. Naming of Enzymes – most are named by adding “ase” to the substrate 4. Sometimes an enzyme needs help Helper molecules are called cofactors (or coenzymes) 5. Factors Influencing Enzyme Activity a. Anything that affects the shape of the enzyme (protein) 1. pH, Temperature, Substrate concentratio ...
... 3. Naming of Enzymes – most are named by adding “ase” to the substrate 4. Sometimes an enzyme needs help Helper molecules are called cofactors (or coenzymes) 5. Factors Influencing Enzyme Activity a. Anything that affects the shape of the enzyme (protein) 1. pH, Temperature, Substrate concentratio ...
Types of Organic compounds
... The enzyme is gray, the substrate is green, the non-competitive inhibitor is red and the products are yellow (A) and blue (B). The enzyme has two binding sites, one for the substrate (the active site) and the other for the non-competitive inhibitor (the regulatory site). When the noncompetitive inh ...
... The enzyme is gray, the substrate is green, the non-competitive inhibitor is red and the products are yellow (A) and blue (B). The enzyme has two binding sites, one for the substrate (the active site) and the other for the non-competitive inhibitor (the regulatory site). When the noncompetitive inh ...
B. Enzymes have four features
... -competitive inhibitors bond to the enzymes in their active site so the substrate cannot; they are in direct competition with the substrate for the active site -noncompetitive inhibitors bond to the enzyme somewhere other than the active site; but, their bonding causes a conformational change in the ...
... -competitive inhibitors bond to the enzymes in their active site so the substrate cannot; they are in direct competition with the substrate for the active site -noncompetitive inhibitors bond to the enzyme somewhere other than the active site; but, their bonding causes a conformational change in the ...
Characteristics of enzymes
... Lock and key hypothesis • The substances on which enzymes act are called substrates. • Active sites are depressions or ‘pockets’ on the surface of an enzyme molecule into which the substrate molecule(s) can fitjust like a lock and key. ...
... Lock and key hypothesis • The substances on which enzymes act are called substrates. • Active sites are depressions or ‘pockets’ on the surface of an enzyme molecule into which the substrate molecule(s) can fitjust like a lock and key. ...
Ch 16.4 Enzymes and rest
... Cannot be cleared out of cells (lack of enzyme!) but accumulate and form amyloid aggregates in brain and nervous tissue (lots of beta pleated sheets) ...
... Cannot be cleared out of cells (lack of enzyme!) but accumulate and form amyloid aggregates in brain and nervous tissue (lots of beta pleated sheets) ...
Ch. 5 Enzyme Review
... a. A competitive inhibitor binds to the enzyme outside the active site. b. The action of competitive inhibitors may be reversible or irreversible. c. A noncompetitive inhibitor does not change the shape of the active site. d. When the product of an enzyme or an enzyme sequence acts as its inhibitor, ...
... a. A competitive inhibitor binds to the enzyme outside the active site. b. The action of competitive inhibitors may be reversible or irreversible. c. A noncompetitive inhibitor does not change the shape of the active site. d. When the product of an enzyme or an enzyme sequence acts as its inhibitor, ...
BIOCHEMISTRY (CHEM 360)
... (3) 2,3-BPG interacts with amino acid side chains in a different location than the heme. (4) Binding of oxygen causes conformational changes at locations other than the heme. (5) Hemoglobin undergoes severe steric interactions. ...
... (3) 2,3-BPG interacts with amino acid side chains in a different location than the heme. (4) Binding of oxygen causes conformational changes at locations other than the heme. (5) Hemoglobin undergoes severe steric interactions. ...
ENZYMES: CLASSIFICATION, STRUCTURE
... •The enzyme cannot differentiate between the two compounds •When inhibitor binds, prevents the substrate from binding •Inhibitor can be released by increasing substrate concentration ...
... •The enzyme cannot differentiate between the two compounds •When inhibitor binds, prevents the substrate from binding •Inhibitor can be released by increasing substrate concentration ...
pH and enzymes in cheese making File
... Because enzymes are proteins, they are denatured by heat or some chemicals Denaturing involves a change of shape in the enzyme molecule so that it cannot combine with the substrate Individual enzymes work best at a particular temperature and pH (acidity or alkalinity) ...
... Because enzymes are proteins, they are denatured by heat or some chemicals Denaturing involves a change of shape in the enzyme molecule so that it cannot combine with the substrate Individual enzymes work best at a particular temperature and pH (acidity or alkalinity) ...
Assignment 5 Bioenergy/ Photosynthesis
... energy by being able to bind to the substrate and then change their conformation to force the reaction. Denaturation of a protein is a process by which some factors, like temperature or pH, affect the shape of a protein by breaking or disrupting the bonds (H-bonds, R-R interactions, or ionic interac ...
... energy by being able to bind to the substrate and then change their conformation to force the reaction. Denaturation of a protein is a process by which some factors, like temperature or pH, affect the shape of a protein by breaking or disrupting the bonds (H-bonds, R-R interactions, or ionic interac ...
Enzymes and Temperature
... changes the shape of the active site. This means that an enzyme-substrate complex cannot form. The proteases pepsin and trypsin are both produced by cells in an inactive form. The acid in the stomach changes the enzymes into their active form. Suggest why these enzymes are first secreted in their in ...
... changes the shape of the active site. This means that an enzyme-substrate complex cannot form. The proteases pepsin and trypsin are both produced by cells in an inactive form. The acid in the stomach changes the enzymes into their active form. Suggest why these enzymes are first secreted in their in ...
Enzyme inhibitor

An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.The binding of an inhibitor can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. Inhibitor binding is either reversible or irreversible. Irreversible inhibitors usually react with the enzyme and change it chemically (e.g. via covalent bond formation). These inhibitors modify key amino acid residues needed for enzymatic activity. In contrast, reversible inhibitors bind non-covalently and different types of inhibition are produced depending on whether these inhibitors bind to the enzyme, the enzyme-substrate complex, or both.Many drug molecules are enzyme inhibitors, so their discovery and improvement is an active area of research in biochemistry and pharmacology. A medicinal enzyme inhibitor is often judged by its specificity (its lack of binding to other proteins) and its potency (its dissociation constant, which indicates the concentration needed to inhibit the enzyme). A high specificity and potency ensure that a drug will have few side effects and thus low toxicity.Enzyme inhibitors also occur naturally and are involved in the regulation of metabolism. For example, enzymes in a metabolic pathway can be inhibited by downstream products. This type of negative feedback slows the production line when products begin to build up and is an important way to maintain homeostasis in a cell. Other cellular enzyme inhibitors are proteins that specifically bind to and inhibit an enzyme target. This can help control enzymes that may be damaging to a cell, like proteases or nucleases. A well-characterised example of this is the ribonuclease inhibitor, which binds to ribonucleases in one of the tightest known protein–protein interactions. Natural enzyme inhibitors can also be poisons and are used as defences against predators or as ways of killing prey.