GSCI 101A - Section 006
... a) arise from magnetized iron. b) have both a north pole and a south pole, without exception. c) arise from electrons orbiting nuclei. d) both b) and c) are correct. d. 54. Many light (as in “not heavy”) radioactive isotopes that undergo radioactive decay do so because a) losing an alpha particle w ...
... a) arise from magnetized iron. b) have both a north pole and a south pole, without exception. c) arise from electrons orbiting nuclei. d) both b) and c) are correct. d. 54. Many light (as in “not heavy”) radioactive isotopes that undergo radioactive decay do so because a) losing an alpha particle w ...
Practice_Final_B
... 25. A household circuit rated at 120 Volts is protected by a fuse rated at 15 amps. What is the maximum number of 100 watt light bulbs which can be lit simultaneously in parallel in this circuit without blowing the fuse? A) 20 B) 4 C) 8 D) 24 E) 18 ...
... 25. A household circuit rated at 120 Volts is protected by a fuse rated at 15 amps. What is the maximum number of 100 watt light bulbs which can be lit simultaneously in parallel in this circuit without blowing the fuse? A) 20 B) 4 C) 8 D) 24 E) 18 ...
The Atomic Theory
... The chemical action of an electric current is directly proportional to the quantity of electricity which passes through a solution. The weights of the substances deposited by the same quantity of electricity are proportional to their chemical equivalents. Stoney (1874) made the hypothesis that there ...
... The chemical action of an electric current is directly proportional to the quantity of electricity which passes through a solution. The weights of the substances deposited by the same quantity of electricity are proportional to their chemical equivalents. Stoney (1874) made the hypothesis that there ...
Unit Description - Honors Chemistry
... Differentiate among the physical states of matter. Classify changes in matter as exothermic or endothermic. Define physical change and list several common physical changes. Define chemical change and list several indications that a chemical change has taken place. Apply the law of conserva ...
... Differentiate among the physical states of matter. Classify changes in matter as exothermic or endothermic. Define physical change and list several common physical changes. Define chemical change and list several indications that a chemical change has taken place. Apply the law of conserva ...
Build your own atom - The Initiating New Science Partnerships in
... currently described as probabilistic volumes (most are familiar with the s, p, d, and f orbitals), and the nucleus is made of nucleons (protons and neutrons) which are themselves each made of three smaller particles, called quarks. The specific combination of these three quarks dictates whether the ...
... currently described as probabilistic volumes (most are familiar with the s, p, d, and f orbitals), and the nucleus is made of nucleons (protons and neutrons) which are themselves each made of three smaller particles, called quarks. The specific combination of these three quarks dictates whether the ...
Study Guide Matter: Building Blocks of the Universe
... * Know the key people in the history of the atom and their contribution to our understanding of the atom. These should be in your lab book conclusion for shoe box atoms. * Know the atomic particles: electron, neutron, and proton. where are they in the atom? What is their charge? What is their mass? ...
... * Know the key people in the history of the atom and their contribution to our understanding of the atom. These should be in your lab book conclusion for shoe box atoms. * Know the atomic particles: electron, neutron, and proton. where are they in the atom? What is their charge? What is their mass? ...
Energy Levels of Helium Nucleus
... and either of the nucleon in the Deuteron nucleus [1]. There are no known solutions of the Schrödinger equation of this nucleus with Yukawa potential. Are there any central potentials with which we can solve the Schrödinger equation for many nuclei such that their ground state wave functions and exc ...
... and either of the nucleon in the Deuteron nucleus [1]. There are no known solutions of the Schrödinger equation of this nucleus with Yukawa potential. Are there any central potentials with which we can solve the Schrödinger equation for many nuclei such that their ground state wave functions and exc ...
Radioactivity - Revision World
... Rutherford Scattering Rutherford proposed a model of the atom to consist of: A heavy, positively charged Nucleus at the centre, with a much lighter, negatively charged electron field in orbit around it. The Rutherford Scattering experiment consists of a piece of gold foil, which is bombarded with p ...
... Rutherford Scattering Rutherford proposed a model of the atom to consist of: A heavy, positively charged Nucleus at the centre, with a much lighter, negatively charged electron field in orbit around it. The Rutherford Scattering experiment consists of a piece of gold foil, which is bombarded with p ...
Chapter 11 Evidence for Strong and Weak Forces in Nuclei
... Consider the natural decay of Tritium 3H1 with a half-life of 12.33 years into 3He2 and an escaping electron which then is captured as an orbiting electron of the Helium nucleus. This situation is clearly one where the exact same amount and number of objects are involved, i.e. three protons and thre ...
... Consider the natural decay of Tritium 3H1 with a half-life of 12.33 years into 3He2 and an escaping electron which then is captured as an orbiting electron of the Helium nucleus. This situation is clearly one where the exact same amount and number of objects are involved, i.e. three protons and thre ...
Ch. 4
... The nucleus is the tiny positive core of the atom which contains most of the mass of the atom. The proton (p+) is the positively (1+) charged particle found in the nucleus of the atom. It has a relative mass of one. The neutron (no) is the particle with no charge (0) found in the nucleus of the atom ...
... The nucleus is the tiny positive core of the atom which contains most of the mass of the atom. The proton (p+) is the positively (1+) charged particle found in the nucleus of the atom. It has a relative mass of one. The neutron (no) is the particle with no charge (0) found in the nucleus of the atom ...
StandardModel
... What will happen if we try to bring it all together ? ----This synthesis of current knowledge, without any doubt is known as ---“The Standard Model ” A glimpse into the Big Bang! It is clear from the figure that all 4 forces were created from a super force during the Big-Bang! In reverse way, we are ...
... What will happen if we try to bring it all together ? ----This synthesis of current knowledge, without any doubt is known as ---“The Standard Model ” A glimpse into the Big Bang! It is clear from the figure that all 4 forces were created from a super force during the Big-Bang! In reverse way, we are ...
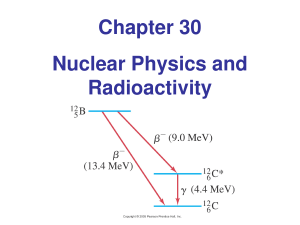
Chapter 30 Nuclear Physics and Radioactivity
... Binding Energy and Nuclear Forces The total mass of a stable nucleus is always less than the sum of the masses of its separate pieces; the protons and neutrons. Where has the mass gone? Energy, as radiation or kinetic energy, is released during formation of a nucleus by ‘fusion’ of smaller nucl ...
... Binding Energy and Nuclear Forces The total mass of a stable nucleus is always less than the sum of the masses of its separate pieces; the protons and neutrons. Where has the mass gone? Energy, as radiation or kinetic energy, is released during formation of a nucleus by ‘fusion’ of smaller nucl ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.