Static Electricity
... • The electromagnetic force is what binds electrons to the nuclei of atoms • Responsible for all of chemistry • When we liberate electrons from individual atoms, we can make them do work for us • So, the topic is extremely important if we are to understand the world around us ...
... • The electromagnetic force is what binds electrons to the nuclei of atoms • Responsible for all of chemistry • When we liberate electrons from individual atoms, we can make them do work for us • So, the topic is extremely important if we are to understand the world around us ...
Elements Elements (cont.) Elements (cont.)
... charge and make the atom electrically neutral • Assume “Plum Pudding Model” electrons suspended in a positively charged electric field ...
... charge and make the atom electrically neutral • Assume “Plum Pudding Model” electrons suspended in a positively charged electric field ...
atomic number
... to answer these questions, Rutherford proposed that there was another particle in the nucleus – called a neutron neutrons have no charge and a mass of 1 amu – the masses of the proton and neutron are both approximately 1 amu This lead to the modern model of the atom. ...
... to answer these questions, Rutherford proposed that there was another particle in the nucleus – called a neutron neutrons have no charge and a mass of 1 amu – the masses of the proton and neutron are both approximately 1 amu This lead to the modern model of the atom. ...
Student Exploration Sheet: Growing Plants
... because it is a mixture of many substances. Water is a pure substance, but it contains two different kinds of atoms: oxygen and hydrogen. Iron is an element because it is composed of one kind of atom. Gizmo Warm-up Atoms are tiny particles of matter that are made up of three particles: protons, neut ...
... because it is a mixture of many substances. Water is a pure substance, but it contains two different kinds of atoms: oxygen and hydrogen. Iron is an element because it is composed of one kind of atom. Gizmo Warm-up Atoms are tiny particles of matter that are made up of three particles: protons, neut ...
Rutherford Scattering
... Increase the energy of the incoming α particle, the distance of closest approach will be smaller. At some rm (penetration) the results from scattering experiment will not agree with Rutherford’s prediction and that rm with give the nuclear size. Example: For a alpha particle of 7.7 MeV, the radius o ...
... Increase the energy of the incoming α particle, the distance of closest approach will be smaller. At some rm (penetration) the results from scattering experiment will not agree with Rutherford’s prediction and that rm with give the nuclear size. Example: For a alpha particle of 7.7 MeV, the radius o ...
Fundamentals of Atomic Structure PowerPoint
... The electron was discovered by J.J. Thomson through his study of the cathode-ray tube. His studies also resulted in the determination of the charge-to-mass ratio of an electron. e/m = -1.76 x 108 C/g A new atomic model was developed called the plum pudding model. In 1909, Robert Millikan performed a ...
... The electron was discovered by J.J. Thomson through his study of the cathode-ray tube. His studies also resulted in the determination of the charge-to-mass ratio of an electron. e/m = -1.76 x 108 C/g A new atomic model was developed called the plum pudding model. In 1909, Robert Millikan performed a ...
Alpha Beta Fission Fusion
... electrons around an atom's nucleus. In 1896, Henri Becquerel expanded the field of chemistry to include nuclear changes when he discovered that uranium emitted radiation. Soon after Becquerel's discovery, Marie Sklodowska Curie began studying radioactivity and completed much of the pioneering work o ...
... electrons around an atom's nucleus. In 1896, Henri Becquerel expanded the field of chemistry to include nuclear changes when he discovered that uranium emitted radiation. Soon after Becquerel's discovery, Marie Sklodowska Curie began studying radioactivity and completed much of the pioneering work o ...
Atoms: The Building Blocks of Matter
... All matter is composed of extremely small particles called atoms. Atoms of a given element are identical in size, mass, & other properties. Atoms cannot be subdivided, created, or destroyed. Atoms of different elements combine in simple whole number ratios to form chemical compounds. In chemical rea ...
... All matter is composed of extremely small particles called atoms. Atoms of a given element are identical in size, mass, & other properties. Atoms cannot be subdivided, created, or destroyed. Atoms of different elements combine in simple whole number ratios to form chemical compounds. In chemical rea ...
1 - kurtniedenzu
... b. Protons c. Valence electrons d. Orbital electrons 31. The amount of energy required to remove the most loosely bound electron from an atom in the gaseous phase is called: a. Kinetic energy b. Potential energy c. Ionization energy d. Electron affinity 32. What is the maximum number of sublevels in ...
... b. Protons c. Valence electrons d. Orbital electrons 31. The amount of energy required to remove the most loosely bound electron from an atom in the gaseous phase is called: a. Kinetic energy b. Potential energy c. Ionization energy d. Electron affinity 32. What is the maximum number of sublevels in ...
Lecture Summary June 10th
... (1) Each element is made up of tiny particles called atoms. (2) The atoms of a given element are identical; the atoms of different elements are different in some fundamental ways. (3) Chemical compounds are formed when atoms of different elements combine with each other. A given compound always has ...
... (1) Each element is made up of tiny particles called atoms. (2) The atoms of a given element are identical; the atoms of different elements are different in some fundamental ways. (3) Chemical compounds are formed when atoms of different elements combine with each other. A given compound always has ...
From ancient Greece to Nobel prize: a Higgs timeline
... United States propose that protons and neutrons well as invisible and indivisible particles called are comprised of quarks. atoms. 1974: The Standard Model of physics is devised: a theory that everything in the Universe is made up of 12 building-block particles governed by four fundamental forces. T ...
... United States propose that protons and neutrons well as invisible and indivisible particles called are comprised of quarks. atoms. 1974: The Standard Model of physics is devised: a theory that everything in the Universe is made up of 12 building-block particles governed by four fundamental forces. T ...
Atomic nucleus
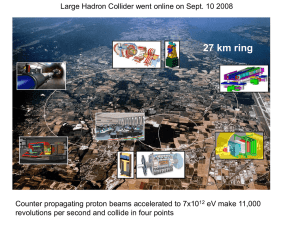
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.