Nucleon number
... Define relative atomic mass and relative molecular mass based on the C-12 scale. Sketch and explain the function of the following main components of a simple mass spectrum: Analyze mass spectrum of an element. Name cations, anions and salt according to the IUPAC. ...
... Define relative atomic mass and relative molecular mass based on the C-12 scale. Sketch and explain the function of the following main components of a simple mass spectrum: Analyze mass spectrum of an element. Name cations, anions and salt according to the IUPAC. ...
Review & Closure - Little Shop of Physics
... Many speculative plans for spaceships capable of inters travel have been developed over the years. Nearly all are ered by the fusion of light nuclei, one of a very few p sources capable of providing the incredibly large ene required. A typical design for a fusion-powered craft h 1.7 * 106 kg ship br ...
... Many speculative plans for spaceships capable of inters travel have been developed over the years. Nearly all are ered by the fusion of light nuclei, one of a very few p sources capable of providing the incredibly large ene required. A typical design for a fusion-powered craft h 1.7 * 106 kg ship br ...
Midterm Review Sheet
... listing the elements in the periodic table by increasing atomic number. 13. Bohr - developed an atomic model for hydrogen, which explained its emission spectra; orbits, ground state, excited state, energy levels; Lyman, Balmer, Paschen, and Brackett series in the hydrogen atom. 14. de Broglie - dete ...
... listing the elements in the periodic table by increasing atomic number. 13. Bohr - developed an atomic model for hydrogen, which explained its emission spectra; orbits, ground state, excited state, energy levels; Lyman, Balmer, Paschen, and Brackett series in the hydrogen atom. 14. de Broglie - dete ...
Document
... 1. Positive nucleus…negative electrons. 2. Electrons are all the same. 3. Nucleus: protons and neutrons…blah, blah. 4. Atoms are neutral until they become ions…….. ...
... 1. Positive nucleus…negative electrons. 2. Electrons are all the same. 3. Nucleus: protons and neutrons…blah, blah. 4. Atoms are neutral until they become ions…….. ...
Chapter 7 Periodic Properties of the Elements
... The electron is attracted to the nucleus, but repelled by electrons that shield or screen it from the full nuclear charge. ...
... The electron is attracted to the nucleus, but repelled by electrons that shield or screen it from the full nuclear charge. ...
Chemistry - El Camino College
... 2. _________ Bonds are strong chemical bonds between atoms that result from the _______ of electrons in their outer orbitals. Molecules with covalent bonds are represented 2 ways: a. ___________ formulas in which each pair of shared electrons is represented by a line (e.g.: O=C=O). b. __________ for ...
... 2. _________ Bonds are strong chemical bonds between atoms that result from the _______ of electrons in their outer orbitals. Molecules with covalent bonds are represented 2 ways: a. ___________ formulas in which each pair of shared electrons is represented by a line (e.g.: O=C=O). b. __________ for ...
for free - Livewire Learning
... through the foil (path 1). 5. This was a confirmation that the gold atoms were mainly space. 6. Some alpha particles were scattered appreciably (path 2) and a very few were deflected through more than 90o. 7. Rutherford explained that if a positively charged alpha particle came in the vicinity of a ...
... through the foil (path 1). 5. This was a confirmation that the gold atoms were mainly space. 6. Some alpha particles were scattered appreciably (path 2) and a very few were deflected through more than 90o. 7. Rutherford explained that if a positively charged alpha particle came in the vicinity of a ...
Summary 1b.1 - Uddingston Grammar School
... If there is so much solute dissolved in the solution that no more can dissolve we say that we have a saturated solution. If we add more solute to a saturated solution it will not dissolve. It will simply lie at the bottom of the beaker. Even stirring will not make any more dissolve. The solution ...
... If there is so much solute dissolved in the solution that no more can dissolve we say that we have a saturated solution. If we add more solute to a saturated solution it will not dissolve. It will simply lie at the bottom of the beaker. Even stirring will not make any more dissolve. The solution ...
Station 1 Answer PowerPoint
... Ernest Rutherford - Discovered the nucleus using gold foil experiment James Chadwick - Discovered the neutron Henri Becquerel - discovered radiation emitted by Uranium Marie Curie - discovered two other elements that emitted radiation (Polonium and Radium) Niels Bohr - Proposed energy levels and the ...
... Ernest Rutherford - Discovered the nucleus using gold foil experiment James Chadwick - Discovered the neutron Henri Becquerel - discovered radiation emitted by Uranium Marie Curie - discovered two other elements that emitted radiation (Polonium and Radium) Niels Bohr - Proposed energy levels and the ...
Name: Date: Period: Who is the Father of Atomic Theory? What
... 3. How many electrons can exist on the following energy levels? 2nd: 3rd: 4th: 4. What characteristic of an atom tells us how many protons reside in the nucleus and helps to indentify the element? 5. Fill in the blanks. – “All atoms with the number of are of the element.” 6. What do we call two atom ...
... 3. How many electrons can exist on the following energy levels? 2nd: 3rd: 4th: 4. What characteristic of an atom tells us how many protons reside in the nucleus and helps to indentify the element? 5. Fill in the blanks. – “All atoms with the number of are of the element.” 6. What do we call two atom ...
Quarter 1 Unit 3 Radioactivitypptx
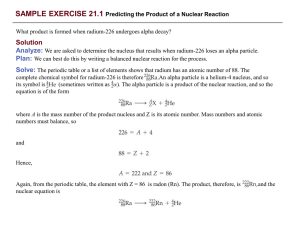
... 2. Alpha- gives off alpha particle which is 2 protons and 2 neutrons. It reduces the atomic number by 2 and the mass by 4 so becomes a new element Beta- a neutron becomes a proton and an electron and gives off the electron, it adds 1 to the atomic number but leaves the mass number the same so a new ...
... 2. Alpha- gives off alpha particle which is 2 protons and 2 neutrons. It reduces the atomic number by 2 and the mass by 4 so becomes a new element Beta- a neutron becomes a proton and an electron and gives off the electron, it adds 1 to the atomic number but leaves the mass number the same so a new ...
Nuclear Magnetic Resonance Spectroscopy
... ¾ The number of signals shows how many different kinds of protons are present. ¾ The location of the signals shows how shielded or deshielded the proton is. ¾ The intensity of the signal shows the number of protons of that type. ¾ Signal splitting shows the number of protons ...
... ¾ The number of signals shows how many different kinds of protons are present. ¾ The location of the signals shows how shielded or deshielded the proton is. ¾ The intensity of the signal shows the number of protons of that type. ¾ Signal splitting shows the number of protons ...
Practice Final fall 2012
... 2. The object in the sky that lies very nearly on an extension of the earth's axis is A. the sun. B. Orion. C. Mercury. D. Polaris 3. In which one or more of the following is the earth assumed to be the center of the universe? A. the Ptolemaic system B. the Copernican system C. Kepler's laws of plan ...
... 2. The object in the sky that lies very nearly on an extension of the earth's axis is A. the sun. B. Orion. C. Mercury. D. Polaris 3. In which one or more of the following is the earth assumed to be the center of the universe? A. the Ptolemaic system B. the Copernican system C. Kepler's laws of plan ...
Nuclear Radiation1516
... Nuclear Chemistry “Nuclear radiation is in the form of elementary particles emitted by an atomic nucleus, as alpha rays, beta rays, or gamma rays, produced by decay of radioactive substances or by nuclear fission.” ...
... Nuclear Chemistry “Nuclear radiation is in the form of elementary particles emitted by an atomic nucleus, as alpha rays, beta rays, or gamma rays, produced by decay of radioactive substances or by nuclear fission.” ...
Atomic Theory Practice Test
... Identify the letter of the choice that best completes the statement or answers the question. ____ 18. The electrons involved in the formation of a chemical bond are called a. dipoles. c. Lewis electrons. b. s electrons. d. valence electrons. ____ 19. In a chemical bond, the link between atoms result ...
... Identify the letter of the choice that best completes the statement or answers the question. ____ 18. The electrons involved in the formation of a chemical bond are called a. dipoles. c. Lewis electrons. b. s electrons. d. valence electrons. ____ 19. In a chemical bond, the link between atoms result ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.