Practice Final Spring 2016
... 2. The object in the sky that lies very nearly on an extension of the earth's axis is A. the sun. B. Orion. C. Mercury. D. Polaris 3. In which one or more of the following is the earth assumed to be the center of the universe? A. the Ptolemaic system B. the Copernican system C. Kepler's laws of plan ...
... 2. The object in the sky that lies very nearly on an extension of the earth's axis is A. the sun. B. Orion. C. Mercury. D. Polaris 3. In which one or more of the following is the earth assumed to be the center of the universe? A. the Ptolemaic system B. the Copernican system C. Kepler's laws of plan ...
Physics, Chapter 44: Stable Nuclei
... the nuclear force. Thus we find that the more massive nuclei which have large values of Z must have more neutrons per proton than the lighter nuclei. It is to be expected that the ideas and concepts that proved so effective in determining the electronic structure of atoms should be carried over into ...
... the nuclear force. Thus we find that the more massive nuclei which have large values of Z must have more neutrons per proton than the lighter nuclei. It is to be expected that the ideas and concepts that proved so effective in determining the electronic structure of atoms should be carried over into ...
TEST on Atomic Structure
... _____ 26) Which of the following is true about subatomic particles? a. Electrons have no charge and have almost no mass. b. Protons are negatively charged and the lightest subatomic particle. c. Neutrons have a negative charge and are the lightest subatomic particle. d. Electrons have almost no mass ...
... _____ 26) Which of the following is true about subatomic particles? a. Electrons have no charge and have almost no mass. b. Protons are negatively charged and the lightest subatomic particle. c. Neutrons have a negative charge and are the lightest subatomic particle. d. Electrons have almost no mass ...
Unit 2 Spiraling
... Show work and box answers. All answers should have units and be to the correct number of significant figures: 9. How many grams are there in 1.50 moles of Na (sodium metal)? How many atoms in the same amount? 10. How many grams are there in 0.00150 moles of H2O? How many molecules in the same amount ...
... Show work and box answers. All answers should have units and be to the correct number of significant figures: 9. How many grams are there in 1.50 moles of Na (sodium metal)? How many atoms in the same amount? 10. How many grams are there in 0.00150 moles of H2O? How many molecules in the same amount ...
Midterm Review 4
... 17. The sum of the protons and neutrons in a lithium atom is: a. 12 b. 13 c. 9 d. 7 18. The electron was discovered by a. Bohr b. Thompson c. Maxwell d. Dalton 19. The number of neutrons in a fluorine atom is: a. 19 b. 10 c. 9 d. 18.998 20. The net charge on any atom is: a. positive b. negative c. n ...
... 17. The sum of the protons and neutrons in a lithium atom is: a. 12 b. 13 c. 9 d. 7 18. The electron was discovered by a. Bohr b. Thompson c. Maxwell d. Dalton 19. The number of neutrons in a fluorine atom is: a. 19 b. 10 c. 9 d. 18.998 20. The net charge on any atom is: a. positive b. negative c. n ...
2005 - The Physics Teacher
... close to a high voltage and would reel away at high speed. It would be an artificial particle accelerator. Potentially such apparatus might allow physicists to break up all atomic nuclei at will. (Adapted from “The Fly in the Cathedral” Brian Cathcart; 2004) (i) What is the structure of an alpha par ...
... close to a high voltage and would reel away at high speed. It would be an artificial particle accelerator. Potentially such apparatus might allow physicists to break up all atomic nuclei at will. (Adapted from “The Fly in the Cathedral” Brian Cathcart; 2004) (i) What is the structure of an alpha par ...
Spring Benchmark Exam
... B Neutrons effectively block the protons and keep them far apart to prevent repulsion. C Electrostatic forces between neutrons and protons hold the nucleus together. D Nuclear forces overcome repulsive forces between protons in the nucleus. ...
... B Neutrons effectively block the protons and keep them far apart to prevent repulsion. C Electrostatic forces between neutrons and protons hold the nucleus together. D Nuclear forces overcome repulsive forces between protons in the nucleus. ...
D. Gravitational, Electric, and Magnetic Fields
... • use appropriate terminology related to fields, including, but not limited to: forces, potential energies, potential, and exchange particles • analyse, and solve problems relating to, Newton’s law of universal gravitation and circular motion (e.g., with respect to satellite orbits, black holes, d ...
... • use appropriate terminology related to fields, including, but not limited to: forces, potential energies, potential, and exchange particles • analyse, and solve problems relating to, Newton’s law of universal gravitation and circular motion (e.g., with respect to satellite orbits, black holes, d ...
2 - Physics with Ms. Selman
... uranium with neutrons, producing new radioactive isotopes. In 1939, German scientists Otto Hahn and Fritz Strassmann found that barium was produced by bombarding uranium with neutrons. Lisa Meitner and Otto Frisch proposed that the neutrons caused the uranium to divide into two smaller nuclei, accom ...
... uranium with neutrons, producing new radioactive isotopes. In 1939, German scientists Otto Hahn and Fritz Strassmann found that barium was produced by bombarding uranium with neutrons. Lisa Meitner and Otto Frisch proposed that the neutrons caused the uranium to divide into two smaller nuclei, accom ...
Name_________________________________ Period: 6 7 8 Date
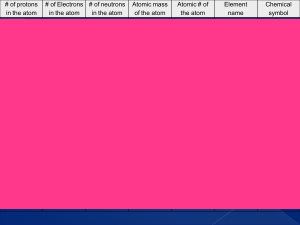
... 22. When an atom gains an electron and becomes negatively charged, we refer to it as a(n) A) negative ion (because it has more electrons than protons) 23. A molecule consisting of two hydrogen atoms joined in a covalent bond would be written as _. B) H2 24. For a neutral atom of an element, the numb ...
... 22. When an atom gains an electron and becomes negatively charged, we refer to it as a(n) A) negative ion (because it has more electrons than protons) 23. A molecule consisting of two hydrogen atoms joined in a covalent bond would be written as _. B) H2 24. For a neutral atom of an element, the numb ...
Modern Physics MC Practice Answers
... + energy. The fragments created are not always the same and there is a statistical probability of which fragments can be created. The reaction provided in this problem is the most probable but other elements can be formed such as the following U–235 fission reaction: U-235 + n Zr-94 + Te-139 + 3n ...
... + energy. The fragments created are not always the same and there is a statistical probability of which fragments can be created. The reaction provided in this problem is the most probable but other elements can be formed such as the following U–235 fission reaction: U-235 + n Zr-94 + Te-139 + 3n ...
Unit 2 Part I PowerPoint
... Typically found in heavier nuclei and the means to achieve stability is to reduce mass Nuclei shed mass in the form of a helium nucleus to become more stable Helium nucleus that is released is ionized and called and Alpha Particle ...
... Typically found in heavier nuclei and the means to achieve stability is to reduce mass Nuclei shed mass in the form of a helium nucleus to become more stable Helium nucleus that is released is ionized and called and Alpha Particle ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.