Preview of Period 3: Electromagnetic Waves – Radiant Energy II
... How can electromagnetic waves transfer energy and information? ...
... How can electromagnetic waves transfer energy and information? ...
Example Midterm
... which the electrons are leaving and the negative plate is the plate to which they are heading. As you know, this will slow the electrons down, and if he turns the voltage up high enough, it will slow them to a complete stop and we won’t have emitted electrons. In such a case, we have KE = e · ∆V . D ...
... which the electrons are leaving and the negative plate is the plate to which they are heading. As you know, this will slow the electrons down, and if he turns the voltage up high enough, it will slow them to a complete stop and we won’t have emitted electrons. In such a case, we have KE = e · ∆V . D ...
Glossary - Angelfire
... opposed to a "SCALAR" which only has size). Examples of vectors are "FORCE", "VELOCITY", "ACCELERATION", "WEIGHT", "DISPLACEMENT ...
... opposed to a "SCALAR" which only has size). Examples of vectors are "FORCE", "VELOCITY", "ACCELERATION", "WEIGHT", "DISPLACEMENT ...
Modern Physics
... So the photoelectric effect could be explained by thinking of light as a stream of incoming particles that collided with electrons in the metal. If the photon had enough energy, it could knock the electron free of the metal and send it across the cell to the collector. If photon was too small, it ...
... So the photoelectric effect could be explained by thinking of light as a stream of incoming particles that collided with electrons in the metal. If the photon had enough energy, it could knock the electron free of the metal and send it across the cell to the collector. If photon was too small, it ...
Solutions to the 2017 Sample Exam Paper
... of the photon in kg m s-1 , the answer would then need to be converted to electron volts. Alternatively, the answer to 16a could be used with the 4.14 x 10-15 eV s value for h. So E = 4.14 x 10-15 x 3.0 x 108 / (4.86 x 10-11) (1)= 25571 eV = 26 keV. (1) Note the question says to three sig figs, but ...
... of the photon in kg m s-1 , the answer would then need to be converted to electron volts. Alternatively, the answer to 16a could be used with the 4.14 x 10-15 eV s value for h. So E = 4.14 x 10-15 x 3.0 x 108 / (4.86 x 10-11) (1)= 25571 eV = 26 keV. (1) Note the question says to three sig figs, but ...
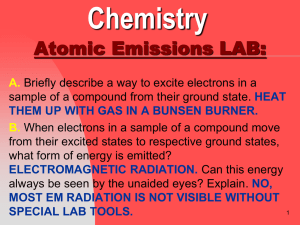
Atomic Emissions LAB Questions
... EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have an emission spectrum with more than one color of light? A SAMPLE HAS MANY ATOMS; EACH ELECTRON IN EACH ATOM WILL MOVE ...
... EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have an emission spectrum with more than one color of light? A SAMPLE HAS MANY ATOMS; EACH ELECTRON IN EACH ATOM WILL MOVE ...
Chapter 7
... • The goal of QM is to solve the Schrodinger Eqn, H Ψ = E Ψ; i.e. find Ψ = atomic orbital plus the associated (quantized) energy for these stable states of the electron in the hydrogen atom. • Ψ2 is related to the probability of finding an electron at a particular (x,y,z) location. Ψ2 is called the ...
... • The goal of QM is to solve the Schrodinger Eqn, H Ψ = E Ψ; i.e. find Ψ = atomic orbital plus the associated (quantized) energy for these stable states of the electron in the hydrogen atom. • Ψ2 is related to the probability of finding an electron at a particular (x,y,z) location. Ψ2 is called the ...
File
... – No photoelectrons emitted if the light frequency falls below a certain threshold frequency, even if the intensity is very high – Threshold frequency, ft, depends on material – If light frequency exceeds ft • # of photoelectrons emitted is proportional to light intensity • Maximum kinetic energy of ...
... – No photoelectrons emitted if the light frequency falls below a certain threshold frequency, even if the intensity is very high – Threshold frequency, ft, depends on material – If light frequency exceeds ft • # of photoelectrons emitted is proportional to light intensity • Maximum kinetic energy of ...
CHAPTER 3: The Experimental Basis of Quantum Theory
... enough energy to escape. Secondary emission: The electron gains enough energy by transfer from another high-speed particle that strikes the material from outside. Field emission: A strong external electric field pulls the electron out of the material. Photoelectric effect: Incident light (elec ...
... enough energy to escape. Secondary emission: The electron gains enough energy by transfer from another high-speed particle that strikes the material from outside. Field emission: A strong external electric field pulls the electron out of the material. Photoelectric effect: Incident light (elec ...
Electron Notes
... used on the walk up. When walking up steps you must exert exactly the specific amount of energy needed to reach the next step. Your steps on steps are quantized, you cannot step between them. ...
... used on the walk up. When walking up steps you must exert exactly the specific amount of energy needed to reach the next step. Your steps on steps are quantized, you cannot step between them. ...
Nov 2009 - Vicphysics
... for A is one wavelength longer than that for B (1). The wavelength = 496 nm. (1) [ /3, %] 5. EK max: The maximum kinetic energy of the electrons (1) ejected from the metal surface as measured by the voltmeter, Vs, when the current, as measured by the microammeter, A, first reads zero. f: The specifi ...
... for A is one wavelength longer than that for B (1). The wavelength = 496 nm. (1) [ /3, %] 5. EK max: The maximum kinetic energy of the electrons (1) ejected from the metal surface as measured by the voltmeter, Vs, when the current, as measured by the microammeter, A, first reads zero. f: The specifi ...
Honors Midterm Review – 2015-16
... _________ responsible for the uncertainty principle which states that it is impossible to know (with any great degree of certainty) both the location and velocity of an electron) _________ responsible for the planetary model of the atom, where electrons traveled in distinct paths around the nucleus ...
... _________ responsible for the uncertainty principle which states that it is impossible to know (with any great degree of certainty) both the location and velocity of an electron) _________ responsible for the planetary model of the atom, where electrons traveled in distinct paths around the nucleus ...