If light is a wave…
... dark lines. These lines are caused by the Sun's atmosphere absorbing light at certain wavelengths, causing the intensity of the light at this wavelength to drop and appear dark. The atoms and molecules in a gas will absorb only certain wavelengths of light. The pattern of these lines is unique to ea ...
... dark lines. These lines are caused by the Sun's atmosphere absorbing light at certain wavelengths, causing the intensity of the light at this wavelength to drop and appear dark. The atoms and molecules in a gas will absorb only certain wavelengths of light. The pattern of these lines is unique to ea ...
1B11 Foundations of Astronomy Star names and magnitudes
... atom or ion, an electron can be moved up to a higher level for a short while, before it falls back down to the ground state. ...
... atom or ion, an electron can be moved up to a higher level for a short while, before it falls back down to the ground state. ...
Misc. Ch 27 Topics
... • 1895 – Wilhelm Roentgen noticed a fluorescent screen glowed when it was several meters away from electric discharges of gases and even with cardboard in between • This must be some mysterious radiation • Called them x-rays • Traveled at the speed of light • Could not be deflected by electric or ma ...
... • 1895 – Wilhelm Roentgen noticed a fluorescent screen glowed when it was several meters away from electric discharges of gases and even with cardboard in between • This must be some mysterious radiation • Called them x-rays • Traveled at the speed of light • Could not be deflected by electric or ma ...
Some Q - Department of Chemistry | Oregon State University
... of the red light is 660 nm. What is the speed of the red light? What is the frequency of the red light? What is the energy of one red light photon? What is the energy of one mole of red light photons? A student obtains a blue laser pointer. What is the speed of the blue light? Which has a longer wav ...
... of the red light is 660 nm. What is the speed of the red light? What is the frequency of the red light? What is the energy of one red light photon? What is the energy of one mole of red light photons? A student obtains a blue laser pointer. What is the speed of the blue light? Which has a longer wav ...
PHYSICS 113 Assignment #2 SOLUTIONS Chapter 4 1. What
... intensity is 8max (microns) = 2900 / T (K). a) At T = 5800 K, 8max = 0.5 microns. This is the green part of the optical (visible) spectrum. b) At T = 1 x 106 K, 8max = 0.0029 microns = 2.9 nm (1 nm = 1 x 10-9 m = 0.001 microns). This corresponds to the far UV (or equivalently the “soft” X-ray) part ...
... intensity is 8max (microns) = 2900 / T (K). a) At T = 5800 K, 8max = 0.5 microns. This is the green part of the optical (visible) spectrum. b) At T = 1 x 106 K, 8max = 0.0029 microns = 2.9 nm (1 nm = 1 x 10-9 m = 0.001 microns). This corresponds to the far UV (or equivalently the “soft” X-ray) part ...
Photosynthesis Pt 1 Light
... • The colors that you see are the wavelengths that are reflected, not absorbed. • If chlorophyll is a green pigment, then what wavelength is not being absorbed? ...
... • The colors that you see are the wavelengths that are reflected, not absorbed. • If chlorophyll is a green pigment, then what wavelength is not being absorbed? ...
Document
... The force felt by a charged particle (an electron in this case) in a magnetic field is perpendicular to the direction of the field and to the direction of the particle's velocity. The net effect of this is to cause the particle to spiral around the direction of the field. Since circular motion repre ...
... The force felt by a charged particle (an electron in this case) in a magnetic field is perpendicular to the direction of the field and to the direction of the particle's velocity. The net effect of this is to cause the particle to spiral around the direction of the field. Since circular motion repre ...
modern physics - FIITJEE Jaipur
... In an experiment on photoelectric effect, the emitter and the collector plates are placed at a separation of 10 cm and are connected through an ammeter without any cell. A magnetic field B exists parallel to the plates. The work function of the emitter is 2.39eV and the light incident on it has wave ...
... In an experiment on photoelectric effect, the emitter and the collector plates are placed at a separation of 10 cm and are connected through an ammeter without any cell. A magnetic field B exists parallel to the plates. The work function of the emitter is 2.39eV and the light incident on it has wave ...
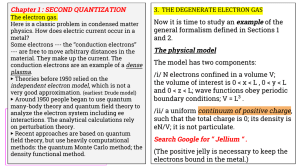
3. THE DEGENERATE ELECTRON GAS Chapter 1 : SECOND QUANTIZATION
... The electron gas. Here is a classic problem in condensed matter physics. How does electric current occur in a metal? Some electrons --- the “conduction electrons” --- are free to move arbitrary distances in the material. They make up the current. The conduction electrons are an example of a dense pl ...
... The electron gas. Here is a classic problem in condensed matter physics. How does electric current occur in a metal? Some electrons --- the “conduction electrons” --- are free to move arbitrary distances in the material. They make up the current. The conduction electrons are an example of a dense pl ...
VCE UNIT 4 SAC
... Analyses and interprets experimental results from a photoelectric effect experiment and explains their significance (DA 1a) Describes, explains and makes links between qualitative and quantitative concepts for photoelectric effect. (DA 1d) Applies ideas and concepts to explanations of new situations ...
... Analyses and interprets experimental results from a photoelectric effect experiment and explains their significance (DA 1a) Describes, explains and makes links between qualitative and quantitative concepts for photoelectric effect. (DA 1d) Applies ideas and concepts to explanations of new situations ...
clicker questions 2
... (B) The voltage has to be greater than Ekin in order to measure a current. Otherwise, the kinetic energy of the electrons is too great, the electrons will leave the setup and there will be no current. (C) As long as the voltage U < Ekin/e, we will measure a current. Otherwise, the positive voltage c ...
... (B) The voltage has to be greater than Ekin in order to measure a current. Otherwise, the kinetic energy of the electrons is too great, the electrons will leave the setup and there will be no current. (C) As long as the voltage U < Ekin/e, we will measure a current. Otherwise, the positive voltage c ...