Schrödinger and Matter Waves
... The quantity |y|2 is interpreted as the probability that the particle can be found at a particular point x and a particular time t ...
... The quantity |y|2 is interpreted as the probability that the particle can be found at a particular point x and a particular time t ...
03 Starlight and Atoms
... Electromagnetic Waves: Oscillating electric and magnetic fields. Changing electric field creates a magnetic field, and vice versa. Speed of electromagnetic waves: c = 3.0 x 108 m/s ...
... Electromagnetic Waves: Oscillating electric and magnetic fields. Changing electric field creates a magnetic field, and vice versa. Speed of electromagnetic waves: c = 3.0 x 108 m/s ...
Solar Electric Power generation
... Direct Conversion of sunlight to energy: Photo-voltaics • Photoelectric effect: • When electromagnetic energy impinges upon a metal surface, electrons are emitted from the surface. • Hertz is often credited with first noticing it (because he published his findings) in 1887 but it was seen by Becque ...
... Direct Conversion of sunlight to energy: Photo-voltaics • Photoelectric effect: • When electromagnetic energy impinges upon a metal surface, electrons are emitted from the surface. • Hertz is often credited with first noticing it (because he published his findings) in 1887 but it was seen by Becque ...
elementary particles history
... radioactive decay series, proposed atomic transmutation in radioactive elements (1902), showed a particles are He nuclei, developed nuclear model of atom (1910) based on a particle scattering, demonstrated artificial transmutation (proton ejected when a collided with N nucleus, measured nuclear size ...
... radioactive decay series, proposed atomic transmutation in radioactive elements (1902), showed a particles are He nuclei, developed nuclear model of atom (1910) based on a particle scattering, demonstrated artificial transmutation (proton ejected when a collided with N nucleus, measured nuclear size ...
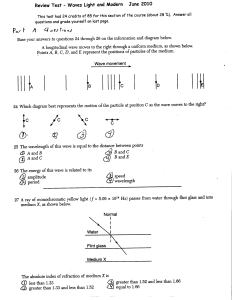
Waves and Modern Physics
... emitted increases, the number of ejected electrons increases and that the max KE of the ejected electrons is determined only by the frequency of the light not the intensity. The latter conclusion could not be explained by classical wave theory. ...
... emitted increases, the number of ejected electrons increases and that the max KE of the ejected electrons is determined only by the frequency of the light not the intensity. The latter conclusion could not be explained by classical wave theory. ...
@ "*pui-,,a"
... substitutionwith units.l lzl 75 Basedon your calculatedvalue of the frequency of the absorbed photon, determine its classi{icationin the electromagnetic spectrum. ...
... substitutionwith units.l lzl 75 Basedon your calculatedvalue of the frequency of the absorbed photon, determine its classi{icationin the electromagnetic spectrum. ...
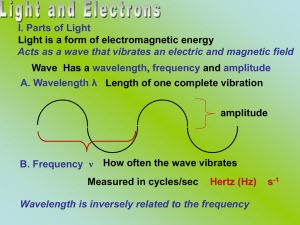
Slide 1
... II. Light as a Particle A. Max Planck Light is released in small units quanta Energy of these units is related to frequency of light E = energy of quantum (J) E = hv v = frequency (s-1) h = Planck’s constant (6.63 x 10-34 J-s) What is the energy of a photon with a wavelength of 236 nm? ...
... II. Light as a Particle A. Max Planck Light is released in small units quanta Energy of these units is related to frequency of light E = energy of quantum (J) E = hv v = frequency (s-1) h = Planck’s constant (6.63 x 10-34 J-s) What is the energy of a photon with a wavelength of 236 nm? ...
CHAPTER 3: The Experimental Basis of Quantum
... What is a photon? Photons move at the speed of light, just like an electromagnetic wave They have zero rest mass and rest energy They carry energy and momentum E=h and p=h/ They can be created and destroyed when radiation is emitted or absorbed They can have particle-like collisions with other pa ...
... What is a photon? Photons move at the speed of light, just like an electromagnetic wave They have zero rest mass and rest energy They carry energy and momentum E=h and p=h/ They can be created and destroyed when radiation is emitted or absorbed They can have particle-like collisions with other pa ...
Quantum Theory
... if v=10,000 m/s, me = 9x10-31 kg and h= 6.6 x 10-34 Joules; the wavelength of the electron is 7 nanometres; the higher the velocity, the shorter the wavelength, so electron microscopes can see things smaller than optical microscopes (wavelength 400-900 nm) ...
... if v=10,000 m/s, me = 9x10-31 kg and h= 6.6 x 10-34 Joules; the wavelength of the electron is 7 nanometres; the higher the velocity, the shorter the wavelength, so electron microscopes can see things smaller than optical microscopes (wavelength 400-900 nm) ...
Heisenberg`s uncertainty principle
... properties, like position and speed, cannot both be known to arbitrary precision: the more precisely one property is known, the less precisely the other can be known. This statement is known as the uncertainty principle. The uncertainty principle isn't a statement about the accuracy of our measuring ...
... properties, like position and speed, cannot both be known to arbitrary precision: the more precisely one property is known, the less precisely the other can be known. This statement is known as the uncertainty principle. The uncertainty principle isn't a statement about the accuracy of our measuring ...
Chapter 5 - Taylor County Schools
... • The wave model of light cannot explain all of light’s characteristics. • An example is the photoelectric effect , when electrons are emitted from a metal’s surface when light of a certain frequency shines on it (how solar calculators work). ...
... • The wave model of light cannot explain all of light’s characteristics. • An example is the photoelectric effect , when electrons are emitted from a metal’s surface when light of a certain frequency shines on it (how solar calculators work). ...