* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download waves
Speed of light wikipedia , lookup
Electromagnetism wikipedia , lookup
Faster-than-light wikipedia , lookup
Circular dichroism wikipedia , lookup
History of optics wikipedia , lookup
Photon polarization wikipedia , lookup
First observation of gravitational waves wikipedia , lookup
Time in physics wikipedia , lookup
Thomas Young (scientist) wikipedia , lookup
Diffraction wikipedia , lookup
Double-slit experiment wikipedia , lookup
Electromagnetic radiation wikipedia , lookup
Matter wave wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
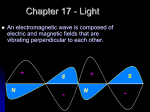
The Godfather is Released (1972) Reading for Monday HOMEWORK – DUE Monday 3/20/17 WS 11 (Worksheet): (from course website) Lab Today/Tomorrow WS 10 (Worksheet): (from course website) CH 6 Extra Credit HOMEWORK – DUE Wednesday 3/22/17 Chapter 8 sections 1-2 EXP 9 Collect EXP 10 data Lab Wednesday/Thursday Finish EXP 10 The Nature of Light: Its Wave Nature Light is a form of electromagnetic radiation made of perpendicular waves, one for the electric field and one for the magnetic field The Nature of Light: Its Wave Nature Light is a form of electromagnetic radiation made of perpendicular waves, one for the electric field and one for the magnetic field All electromagnetic waves move through space at the same, constant speed 2.998 x 108 m/s in a vacuum = the speed of light, c Characterizing Waves The amplitude is the height of the wave the distance from node to crest or node to trough Characterizing Waves Node Characterizing Waves The amplitude is the height of the wave the distance from node to crest or node to trough the amplitude is a measure of how intense the light is – the larger the amplitude, the brighter the light Characterizing Waves Characterizing Waves The wavelength (l) is a measure of the distance covered by the wave the distance from one crest to the next (or the distance from one trough to the next, or the distance between alternate nodes) Characterizing Waves Node Characterizing Waves The wavelength (l) is a measure of the distance covered by the wave the distance from one crest to the next (or the distance from one trough to the next, or the distance between alternate nodes) For visible light, the wavelength is related to the color of light Characterizing Waves Characterizing Waves The frequency (n) is the number of waves that pass a point in a given period of time the number of waves = number of cycles units are hertz (Hz) or cycles/second = s−1 1 Hz = 1 s−1 LIGHT!!! wavelength and energy are INVERSELY proportional wavelength energy LIGHT!!! wavelength and energy are INVERSELY proportional wavelength energy LIGHT!!! energy and frequency are DIRECTLY proportional energy frequency Wavelength and Frequency Wavelength and frequency of electromagnetic waves are inversely proportional because the speed of light is constant, if we know wavelength we can find the frequency, and vice versa c nl Calculate the wavelength of red light (nm) with a frequency of 4.62x1014 s−1 2.998 108 m 1s 1 nm 1s 4.621014 1109 m 649 nm Calculate the wavelength (m) of a radio signal with a frequency of 106.5 MHz 2.998 108 m 1 Hz 1 MHz 1 6 1 1s 110 Hz 106.5 MHz 1s 2.815 m Color The color of light is determined by its wavelength or frequency White light is a mixture of all the colors of visible light a spectrum RedOrangeYellowGreenBlueViolet When an object absorbs some of the wavelengths of white light and reflects others, it appears colored the observed color is predominantly the colors reflected Types of Electromagnetic Radiation • Electromagnetic waves are classified by their wavelength Radio waves = l > 0.01 m Microwaves = 1 104 m < l < 1 102 m Infrared (IR) far IR = 1 105 m < l < 1104 m middle IR = 1 106 m < l < 1105 m near IR = 1 107 m < l < 1106 m Visible light = 4 107 m < l < 8 107 m ROY G. BIV Ultraviolet (UV) near UV = 2 107 m < l < 4 107 m far UV = 1 108 m < l < 2 107 m X-rays = 1 10 10 8 m < l < 110 m Gamma rays = l < 11010 m low frequency and energy high frequency and energy Electromagnetic Spectrum Interference The interaction between waves is called interference When waves interact so that they add to make a larger wave it is called constructive interference waves are in-phase Interference The interaction between waves is called interference When waves interact so they cancel each other it is called destructive interference waves are out-of-phase Diffraction When traveling waves encounter an obstacle or opening in a barrier that is about the same size as the wavelength, they bend around it – this is called diffraction Traveling particles do not diffract 2-Slit Interference The diffraction of light through two slits separated by a distance comparable to the wavelength results in an interference pattern of the diffracted waves An interference pattern is a characteristic of all light waves https://www.youtube.com/watch?v=hRFQd_fkzws The Photoelectric Effect Many metals emit electrons when a light shines on them. called the photoelectric effect The Photoelectric Effect The Photoelectric Effect Many metals emit electrons when a light shines on them. called the photoelectric effect Classic wave theory said this effect was due to the light energy being transferred to the electron. The energy of a wave is directly proportional to its amplitude and its frequency If the wavelength of light is made shorter, more electrons should be ejected Light waves’ intensity made brighter, more electrons should be ejected Predicts that if a dim light were used there would be a lag time before electrons were emitted to give the electrons time to absorb enough energy The Photoelectric Effect: The Problem Experiments showed that a minimum frequency was needed before electrons would be emitted called the threshold frequency no dependence on intensity It was observed that high-frequency light from a dim source caused electron emission without any lag time Einstein’s Explanation Einstein proposed that the light energy was delivered to the atoms in packets, called quanta or photons The energy of a photon of light is directly proportional to its frequency and inversely to wavelength the proportionality constant is called Planck’s Constant, (h) and has the value 6.6261034 J s photon E hn hc l Calculate the number of photons in a laser pulse with wavelength 337 nm and total energy 3.83 mJ 1 photon 1103 J 1s 1109 m 3.83 m J 3.37 102 nm 34 8 6.626 10 J s 1 mJ 3.00 10 m 1 nm 6.49x1015 photons What is the frequency of radiation required to supply 1.0 x 102 J of energy from 8.5 x 1027 photons? 1 photon 1.0 102 J 1 6.626 1034 J s 1 8.5 1027 photon 1.8x107 s-1 or 1.8x107 Hz or 18 MHz Ejected Electrons One photon at the threshold frequency gives the electron just enough energy for it to escape the atom binding energy, f When irradiated with a shorter wavelength photon, the electron absorbs more energy than is necessary to escape This excess energy becomes kinetic energy of the ejected electron Kinetic Energy = Ephoton – Ebinding KE = hn − f Suppose a metal will eject electrons from its surface when struck by yellow light. What will happen if the surface is struck with ultraviolet light? 1. No electrons would be ejected. 2. Electrons would be ejected, and they would have the same kinetic energy as those ejected by yellow light. 3. Electrons would be ejected, and they would have greater kinetic energy than those ejected by yellow light. 4. Electrons would be ejected, and they would have lower kinetic energy than those ejected by yellow light.