lewis dot structures
... If there is more than 1 sublevel orbital each is filled with One electron before a second is added to each until The sublevel orbitals are filled. - ex: ...
... If there is more than 1 sublevel orbital each is filled with One electron before a second is added to each until The sublevel orbitals are filled. - ex: ...
Intro to Particle Physics and High Energy Astrophysics
... of the velocity of the charged particles due to relativistic ...
... of the velocity of the charged particles due to relativistic ...
Forms of Energy
... • Elastic potential energy is the energy __________ in compression springs, stretched rubber bands, and bent vaulting poles-all materials that can be forced into a shape that’s different from its natural shape • Gravitational potential energy is the energy an object has when its in an _____________ ...
... • Elastic potential energy is the energy __________ in compression springs, stretched rubber bands, and bent vaulting poles-all materials that can be forced into a shape that’s different from its natural shape • Gravitational potential energy is the energy an object has when its in an _____________ ...
Document
... distribution of energy in blackbody radiation As the temperature increases, the total amount of energy ...
... distribution of energy in blackbody radiation As the temperature increases, the total amount of energy ...
Chemistry 221
... b) Suggest two ions that might form this compound and draw a microscopic sketch of several formula units of this compound. c) How would a sketch representing the molecular compound differ from the one for the ionic compound? ...
... b) Suggest two ions that might form this compound and draw a microscopic sketch of several formula units of this compound. c) How would a sketch representing the molecular compound differ from the one for the ionic compound? ...
III. Atomic Theory
... a) Orbitals: are sub-energy levels of the principal levels predicted by Bohr. b) # orbitals per principal level = n2 e.g. the 3rd energy level hold 18 e- ‘s (2 32). To hold 18 e- ‘s you need 9 ...
... a) Orbitals: are sub-energy levels of the principal levels predicted by Bohr. b) # orbitals per principal level = n2 e.g. the 3rd energy level hold 18 e- ‘s (2 32). To hold 18 e- ‘s you need 9 ...
ionization energies
... notice patterns in the chemical properties of certain elements. • Consider the three metals Li, Na, and K • All 3 metals are soft • All 3 metals are less dense than water • All 3 metals have similar appearance and low melting points • The most interesting feature is that all 3 metals react with the ...
... notice patterns in the chemical properties of certain elements. • Consider the three metals Li, Na, and K • All 3 metals are soft • All 3 metals are less dense than water • All 3 metals have similar appearance and low melting points • The most interesting feature is that all 3 metals react with the ...
Work and Gravitational Potential Energy
... Work-Kinetic Energy Theorem • When work is done by a net force on an object and the only change in the object is its speed, the work done is equal to the change in the object’s kinetic energy Wnet = KEf − KEi = ∆KE ...
... Work-Kinetic Energy Theorem • When work is done by a net force on an object and the only change in the object is its speed, the work done is equal to the change in the object’s kinetic energy Wnet = KEf − KEi = ∆KE ...
toi 40 60
... What is the force that one surface exerts on another when the two rub against each other? ...
... What is the force that one surface exerts on another when the two rub against each other? ...
SCH3U Course Review
... Ionization energies tend to increase with increasing atomic radii decrease with increasing nuclear charge decrease across a period from left to right increase across a period from left to right increase as you go down a family ...
... Ionization energies tend to increase with increasing atomic radii decrease with increasing nuclear charge decrease across a period from left to right increase across a period from left to right increase as you go down a family ...
L01_5342_Sp02
... • Tmax is the energy of the electron emitted from a material surface when light of frequency f is incident. • fo, frequency for zero KE, mat’l spec. • h is Planck’s (a universal) constant h = 6.625E-34 J-sec L1 January 15 ...
... • Tmax is the energy of the electron emitted from a material surface when light of frequency f is incident. • fo, frequency for zero KE, mat’l spec. • h is Planck’s (a universal) constant h = 6.625E-34 J-sec L1 January 15 ...
264-lecture-2015-08-30
... frictionless horizontal surface. If T1 is the period of the motion according to Newtonian mechanics and T2 is the period of the motion according to special relativity then A. T1 = T2 B. T1 > T2 C. T1 < T2 ...
... frictionless horizontal surface. If T1 is the period of the motion according to Newtonian mechanics and T2 is the period of the motion according to special relativity then A. T1 = T2 B. T1 > T2 C. T1 < T2 ...
The$light$that$surrounds$us$ Augusto$Beléndez$
... our!lives.!Clearly,!the!light!emitted!by!the!Sun!plays!a!fundamental!role!in!the!development! of!life!on!Earth!and!it!is!the!main!source!of!energy!for!our!planet.!If!someone!asks!“what! do!we!get!from!the!Sun?,”!we!immediately!answer:!“light!and!warmth”!and!some!might! even! add! “ultraviolet! light ...
... our!lives.!Clearly,!the!light!emitted!by!the!Sun!plays!a!fundamental!role!in!the!development! of!life!on!Earth!and!it!is!the!main!source!of!energy!for!our!planet.!If!someone!asks!“what! do!we!get!from!the!Sun?,”!we!immediately!answer:!“light!and!warmth”!and!some!might! even! add! “ultraviolet! light ...
Document
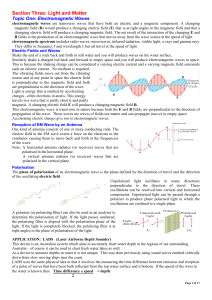
... Light and Sight • The human eye has several parts, including the cornea, the pupil, the iris, the lens, and the retina. • Nearsightedness (use a concave lens) when you can’t see far away and farsightedness (use a convex lens) when you can’t see close up. In both cases, light is not focused on the re ...
... Light and Sight • The human eye has several parts, including the cornea, the pupil, the iris, the lens, and the retina. • Nearsightedness (use a concave lens) when you can’t see far away and farsightedness (use a convex lens) when you can’t see close up. In both cases, light is not focused on the re ...
Chapter 6 Quiz
... ______10. When atoms share electrons, the electrical attraction of an atom for the shared electrons is called the atom's a. electron affinity. b. resonance. c. electronegativity. d. hybridization. ______11. If the atoms that share electrons have an unequal attraction for the electrons, the bond is c ...
... ______10. When atoms share electrons, the electrical attraction of an atom for the shared electrons is called the atom's a. electron affinity. b. resonance. c. electronegativity. d. hybridization. ______11. If the atoms that share electrons have an unequal attraction for the electrons, the bond is c ...