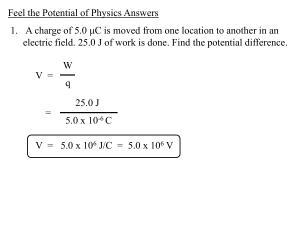
Feel the Potential of Physics Answers
... 3. If an electron is accelerated through a potential difference of 100 V, what is the final speed of the electron? Energy = KE = 1.6021 x 10-17 J KE = 1/2 mv2 ...
... 3. If an electron is accelerated through a potential difference of 100 V, what is the final speed of the electron? Energy = KE = 1.6021 x 10-17 J KE = 1/2 mv2 ...
Apparent Depth
... around 300,000 kilometres per second. At this speed it can go around the world 8 times in one second. ...
... around 300,000 kilometres per second. At this speed it can go around the world 8 times in one second. ...
File
... Answer the following questions regarding light and its interactions with molecules, atoms, and ions. (a) The longest wavelength of light with enough energy to break the Cl-Cl bond in Cl2(g) is 495 nm. (i) Calculate the frequency, in s-1, of the light. (ii) Calculate the energy, in J, of a photon of ...
... Answer the following questions regarding light and its interactions with molecules, atoms, and ions. (a) The longest wavelength of light with enough energy to break the Cl-Cl bond in Cl2(g) is 495 nm. (i) Calculate the frequency, in s-1, of the light. (ii) Calculate the energy, in J, of a photon of ...
Trichromatic Theory of Color Vision
... Q: How many numbers would you need to write down to specify the color of a light source? spectral properties A: It depends on how you “bin” up the spectrum • One number for each spectral “bin”: ...
... Q: How many numbers would you need to write down to specify the color of a light source? spectral properties A: It depends on how you “bin” up the spectrum • One number for each spectral “bin”: ...
Unit f Chapter 3 FORMS OF ENERGY
... What is convection? What type of heat transfer takes place when you burn your hand on a stove? Two atoms absorb thermal energy when joining together to form a molecule. What happens to the thermal energy? Suppose you drop an ice cube into a warm drink, and it melts. How is thermal energy transferre ...
... What is convection? What type of heat transfer takes place when you burn your hand on a stove? Two atoms absorb thermal energy when joining together to form a molecule. What happens to the thermal energy? Suppose you drop an ice cube into a warm drink, and it melts. How is thermal energy transferre ...
The Light Reactions
... • When a photon of light strikes photosystem II, it excites an electron. At the same time an enzyme binds to two water molecules and splits the water into hydrogen ions (H+ or protons) and releases an oxygen atom (O2). Note: This is why water is necessary for photosynthesis to occur and this is whe ...
... • When a photon of light strikes photosystem II, it excites an electron. At the same time an enzyme binds to two water molecules and splits the water into hydrogen ions (H+ or protons) and releases an oxygen atom (O2). Note: This is why water is necessary for photosynthesis to occur and this is whe ...
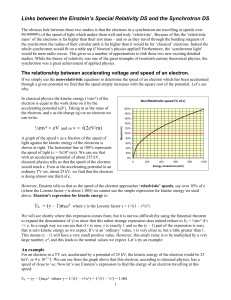
Links between the Einstein`s Special Relativity DS and
... energy. At zero or slow speeds this ratio is effectively 1 and so v is very close to zero (relatively speaking!). If the kinetic energy increases, the second term becomes smaller, and so v increases. As the kinetic energy gets larger and larger, the second term gets smaller and smaller, and so v app ...
... energy. At zero or slow speeds this ratio is effectively 1 and so v is very close to zero (relatively speaking!). If the kinetic energy increases, the second term becomes smaller, and so v increases. As the kinetic energy gets larger and larger, the second term gets smaller and smaller, and so v app ...
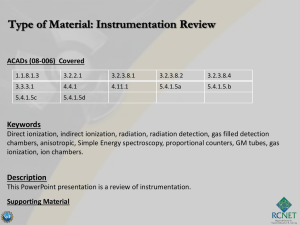
Instrumentation Review
... acquire enough energy to cause secondary ionizations (gas amplification) and increase the charge collected. • These secondary ionizations may cause further ionization • In this region, there is a linear relationship between the number of ion pairs collected and applied voltage. • A charge amplificat ...
... acquire enough energy to cause secondary ionizations (gas amplification) and increase the charge collected. • These secondary ionizations may cause further ionization • In this region, there is a linear relationship between the number of ion pairs collected and applied voltage. • A charge amplificat ...
Magnetic Lenses, Interactions of Electrons with Matter
... Fluorescence yield , w = Z 4 / (Z 4 + c) Much higher for large Z ...
... Fluorescence yield , w = Z 4 / (Z 4 + c) Much higher for large Z ...
Chapter 3
... levels, an electron can have. For each energy level, the Schordinger’s equation also leads to a mathematical expression called an atomic orbital which describes the probability of finding an electron at various locations around the nucleus of. An atomic orbitals is represented pictorially as a regio ...
... levels, an electron can have. For each energy level, the Schordinger’s equation also leads to a mathematical expression called an atomic orbital which describes the probability of finding an electron at various locations around the nucleus of. An atomic orbitals is represented pictorially as a regio ...
6.1 Organizing the Periodic Table
... • Elements are arranged according to atomic number • 7 rows or periods- each corresponds to a principle energy level- the # of elements per period varies because the # of available orbitals increases from energy level to energy level • Elements within a column or group have similar properties • Prop ...
... • Elements are arranged according to atomic number • 7 rows or periods- each corresponds to a principle energy level- the # of elements per period varies because the # of available orbitals increases from energy level to energy level • Elements within a column or group have similar properties • Prop ...