Cell Respiration Stations
... Removes two electrons from QH2 at the QO site Transfers them to two molecules of cytochrome c, a water-soluble electron carrier located within the intermembrane space. The two other electrons passed across the protein quinone, which is reduced to quinol. ...
... Removes two electrons from QH2 at the QO site Transfers them to two molecules of cytochrome c, a water-soluble electron carrier located within the intermembrane space. The two other electrons passed across the protein quinone, which is reduced to quinol. ...
CELLULAR RESPIRATION STATIONS
... Removes two electrons from QH2 at the QO site Transfers them to two molecules of cytochrome c, a water-soluble electron carrier located within the intermembrane space. The two other electrons passed across the protein quinone, which is reduced to quinol. ...
... Removes two electrons from QH2 at the QO site Transfers them to two molecules of cytochrome c, a water-soluble electron carrier located within the intermembrane space. The two other electrons passed across the protein quinone, which is reduced to quinol. ...
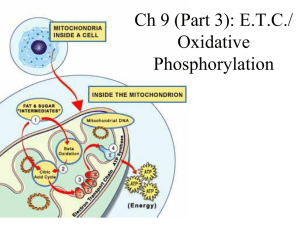
PATHWAYS THAT HARVEST CHEMICAL ENERGY CHAPTER 9
... • Links glycolysis and the citric acid cycle; occurs in the mitochondrial matrix • Pyruvate is oxidized to acetate and CO2 is released • NAD+ is reduced to NADH, capturing energy • Some energy is stored by combining acetate and Coenzyme A (CoA) to form acetyl CoA ...
... • Links glycolysis and the citric acid cycle; occurs in the mitochondrial matrix • Pyruvate is oxidized to acetate and CO2 is released • NAD+ is reduced to NADH, capturing energy • Some energy is stored by combining acetate and Coenzyme A (CoA) to form acetyl CoA ...
Bio426Lecture25Apr3 - NAU jan.ucc.nau.edu web server
... Mitochondrial electron transport oxidizes NAD(P)H, and generates a p.m.f. that is used to produce ATP. O2 is consumed as it acts as the terminal electron acceptor. This production of ATP is called “oxidative phosphorylation” ...
... Mitochondrial electron transport oxidizes NAD(P)H, and generates a p.m.f. that is used to produce ATP. O2 is consumed as it acts as the terminal electron acceptor. This production of ATP is called “oxidative phosphorylation” ...
No Slide Title
... 2. Krebs Cycle (aka. citric acid cycle) Acetyl CoA is broken down completely to CO2. cells use carbon skeletons of intermediates to produce other organic molecules (amino acids). energy harvested per acetyl CoA: 1 ATP (via substrate-level phosphorylation) ...
... 2. Krebs Cycle (aka. citric acid cycle) Acetyl CoA is broken down completely to CO2. cells use carbon skeletons of intermediates to produce other organic molecules (amino acids). energy harvested per acetyl CoA: 1 ATP (via substrate-level phosphorylation) ...
complex I
... How the Proton Gradient Drives Coupled Transport Across the Inner Membrane In mitochondria, many charged small molecules, such as pyruvate, ADP and Pi, are pumped into the matrix from the cytosol, while others, such as ATP must be moved in the opposite direction. •Pyruvate and inorganic phosphate ( ...
... How the Proton Gradient Drives Coupled Transport Across the Inner Membrane In mitochondria, many charged small molecules, such as pyruvate, ADP and Pi, are pumped into the matrix from the cytosol, while others, such as ATP must be moved in the opposite direction. •Pyruvate and inorganic phosphate ( ...
Microbial Metabolism
... chain to some molecule other than oxygen (e.g. NO3, SO4). Inorganic molecules can be oxidized with ATP synthesis by e- transport and chemiosmosis. Fermentation: common anaerobic pathway used by many medically important bacteria. ...
... chain to some molecule other than oxygen (e.g. NO3, SO4). Inorganic molecules can be oxidized with ATP synthesis by e- transport and chemiosmosis. Fermentation: common anaerobic pathway used by many medically important bacteria. ...
studies on the mitochondrial electron transport and atp synthesis
... In the absence or at a low level of ADP the proton flow rate is decreased due to the decreased speed of the phosphorylation. If the proton flow through the F1-F0 complex into the matrix is inhibited, the proton concentration is increased significantly in the intermembrane space and an increased ener ...
... In the absence or at a low level of ADP the proton flow rate is decreased due to the decreased speed of the phosphorylation. If the proton flow through the F1-F0 complex into the matrix is inhibited, the proton concentration is increased significantly in the intermembrane space and an increased ener ...
Biology-1 Sample Questions for Exam Two Facilitated diffusion
... d. is embedded in the outer membrane of the mitochondrion. e. helps transport H+ against the concentration gradient. ...
... d. is embedded in the outer membrane of the mitochondrion. e. helps transport H+ against the concentration gradient. ...
STUDY GUIDE SECTION 7-1 Glycolysis and Fermentation
... 2. ______The starting substance of the Krebs cycle, which is regenerated at the end of the cycle, is a. acetyl CoA b. pyruvic acid c. oxaloacetic acid d. citric acid 3. ______The Krebs cycle a. produces two molecules of CO2. b. produces a six-carbon molecule from six molecules of CO2. c. produces NA ...
... 2. ______The starting substance of the Krebs cycle, which is regenerated at the end of the cycle, is a. acetyl CoA b. pyruvic acid c. oxaloacetic acid d. citric acid 3. ______The Krebs cycle a. produces two molecules of CO2. b. produces a six-carbon molecule from six molecules of CO2. c. produces NA ...
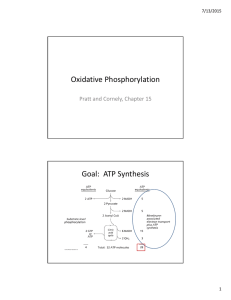
Oxidative Phosphorylation Goal: ATP Synthesis
... • NADH pumps 10 protons when 2 e‐ reduce ½ O2 – 4 protons in Complex I, 4 protons in Complex III, and 2 protons in Complex IV ...
... • NADH pumps 10 protons when 2 e‐ reduce ½ O2 – 4 protons in Complex I, 4 protons in Complex III, and 2 protons in Complex IV ...
fates of pyruvate
... 1)alcohol fermentation – pyruvate converted to ethyl alcohol 2)lactic acid fermentation - pyruvate converted to lactic acid (cheese, yogurt) - Aerobic conditions: Pyruvate enter the mitochondria where it is completely oxidized Pyruvate -> enzyme -> acetyl group + CO2 + NADH ...
... 1)alcohol fermentation – pyruvate converted to ethyl alcohol 2)lactic acid fermentation - pyruvate converted to lactic acid (cheese, yogurt) - Aerobic conditions: Pyruvate enter the mitochondria where it is completely oxidized Pyruvate -> enzyme -> acetyl group + CO2 + NADH ...
METABOLISM - Doctor Jade Main
... Electron Transport Chain • transfers pairs of electrons from entering substrate to final electron acceptoroxygen • electrons are led through series of oxidationreduction reactions before combining with O2 atoms • reactions takes place on inner mitochondrial membrane • only permeable to water, oxyge ...
... Electron Transport Chain • transfers pairs of electrons from entering substrate to final electron acceptoroxygen • electrons are led through series of oxidationreduction reactions before combining with O2 atoms • reactions takes place on inner mitochondrial membrane • only permeable to water, oxyge ...
Biology-1 Sample Questions for Exam Two Facilitated diffusion
... d. is embedded in the outer membrane of the mitochondrion. e. helps transport H+ against the concentration gradient. ...
... d. is embedded in the outer membrane of the mitochondrion. e. helps transport H+ against the concentration gradient. ...
Cell Respiration Worksheet
... In absence of oxygen, get regeneration of NAD+ thru fermentation Realize that some animals (particularly many bacteria) live in anaerobic environments or habitats with very little oxygen. Glycolysis is their main way to get ATP. Glycolysis only produces 2 ATP's by itself (for every molecule of gluco ...
... In absence of oxygen, get regeneration of NAD+ thru fermentation Realize that some animals (particularly many bacteria) live in anaerobic environments or habitats with very little oxygen. Glycolysis is their main way to get ATP. Glycolysis only produces 2 ATP's by itself (for every molecule of gluco ...
Oxidative Phosphorylation - Creighton Chemistry Webserver
... Oxidative Phosphorylation What are the electron carriers? NADH, NADPH (cannot cross inner mito membrane, shuttle their e-) FMN, FAD (directly involved in Ox phos) NADH, NADPH and FADH2 each carry 2eFMN can carry 1 or 2e- ...
... Oxidative Phosphorylation What are the electron carriers? NADH, NADPH (cannot cross inner mito membrane, shuttle their e-) FMN, FAD (directly involved in Ox phos) NADH, NADPH and FADH2 each carry 2eFMN can carry 1 or 2e- ...
Exam 2 Answers
... a. Generates reducing power in the form of NADH and FADH2 Krebs Cycle b. Produces ATP Glycolysis, Krebs Cycle, ETC c. Begins and ends with a 4-carbon molecule Krebs Cycle d. A catabolic pathway that breaks down glucose to pyruvate in the cytoplasm. Glycolysis e. The means by which the three carbons ...
... a. Generates reducing power in the form of NADH and FADH2 Krebs Cycle b. Produces ATP Glycolysis, Krebs Cycle, ETC c. Begins and ends with a 4-carbon molecule Krebs Cycle d. A catabolic pathway that breaks down glucose to pyruvate in the cytoplasm. Glycolysis e. The means by which the three carbons ...
Transport
... o Movement from high to lower concentration areas o Examples include simple diffusion; osmosis and facilitated diffusion o Facilitated diffusion requires a protein carrier or channel Active transport o Energy in the form of ATP is required o Movement from low to high areas of concentration o Example ...
... o Movement from high to lower concentration areas o Examples include simple diffusion; osmosis and facilitated diffusion o Facilitated diffusion requires a protein carrier or channel Active transport o Energy in the form of ATP is required o Movement from low to high areas of concentration o Example ...
Redox reaction during glycolysis
... • NADH+H+ supplies pair of H atoms to the first carrier in the chain, with the NAD+ returning to the matrix. • The hydrogen atoms are split, to release two electrons, which pass from carrier in the chain. • Energy is released as the e- pass from carrier to carrier, and three of these use this energy ...
... • NADH+H+ supplies pair of H atoms to the first carrier in the chain, with the NAD+ returning to the matrix. • The hydrogen atoms are split, to release two electrons, which pass from carrier in the chain. • Energy is released as the e- pass from carrier to carrier, and three of these use this energy ...
Take Home Part 1 - hrsbstaff.ednet.ns.ca
... 13) In liver cells, the inner mitochondrial membranes are about five times the area of the outer mitochondrial membranes. What purpose must this serve? A) It increases the surface for oxidative phosphorylation. B) It allows the liver cell to have fewer mitochondria. C) It increases the surface for ...
... 13) In liver cells, the inner mitochondrial membranes are about five times the area of the outer mitochondrial membranes. What purpose must this serve? A) It increases the surface for oxidative phosphorylation. B) It allows the liver cell to have fewer mitochondria. C) It increases the surface for ...
PowerPoint Presentation - Ch. 6 Cellular Respiration
... energy hill. • What happens to the energy of the electrons as it falls down the electron transport chain? • The energy is used to pump H+ against their gradient which then come back through ATP synthase to generate ATP ...
... energy hill. • What happens to the energy of the electrons as it falls down the electron transport chain? • The energy is used to pump H+ against their gradient which then come back through ATP synthase to generate ATP ...
Vitamins Clinical relevance: homocystinuria: B6 and/or B12 and/or
... roles of Flavins and NAD+/NADH are similar carries electrons reducing equivalents 2 electrons and 2 protons transferred as one electron and one proton simultaneously (2 electrons and 1 proton in case of NAD/NADH) ...
... roles of Flavins and NAD+/NADH are similar carries electrons reducing equivalents 2 electrons and 2 protons transferred as one electron and one proton simultaneously (2 electrons and 1 proton in case of NAD/NADH) ...
Chapter 14-Respiration
... An electrical gradient is also built up across the inner membrane as positive and negative charges are separated. The matrix becomes negative, the inter-membranous space, positive. COMPLEX IV ...
... An electrical gradient is also built up across the inner membrane as positive and negative charges are separated. The matrix becomes negative, the inter-membranous space, positive. COMPLEX IV ...
Electron Transport and ATP Synthesis
... • 10 protons shuttled from matrix to intermembrane space • Makes pH gradient and ion gradient ...
... • 10 protons shuttled from matrix to intermembrane space • Makes pH gradient and ion gradient ...
Oxidative phosphorylation
Oxidative phosphorylation (or OXPHOS in short) is the metabolic pathway in which the mitochondria in cells use their structure, enzymes, and energy released by the oxidation of nutrients to reform ATP. Although the many forms of life on earth use a range of different nutrients, ATP is the molecule that supplies energy to metabolism. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis.During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cells' intermembrane space. These linked sets of proteins are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme called ATP synthase; this process is known as chemiosmosis. This enzyme uses this energy to generate ATP from adenosine diphosphate (ADP), in a phosphorylation reaction. This reaction is driven by the proton flow, which forces the rotation of a part of the enzyme; the ATP synthase is a rotary mechanical motor.Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging (senescence). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.