Chapter 11
... this picture arose because of the response to inhibitors. If an inhibitor blocks electron transfer between two sites (as indicated above for antimycin) then in the presence of an excess of electron donor e.g. NADH the electron carriers to the left of the inhibitory site become reduced while those to ...
... this picture arose because of the response to inhibitors. If an inhibitor blocks electron transfer between two sites (as indicated above for antimycin) then in the presence of an excess of electron donor e.g. NADH the electron carriers to the left of the inhibitory site become reduced while those to ...
Reading Guide for Week 4
... 11. From section 6.4, know that cytochromes are oxidases and are components of the electron transport chain. (Why is iron important?) Why do the oxidase test? 11. Know that eukaryotic mitochondrial electron transport chain and prokaryotic electron transport chains are very similar, but no need to me ...
... 11. From section 6.4, know that cytochromes are oxidases and are components of the electron transport chain. (Why is iron important?) Why do the oxidase test? 11. Know that eukaryotic mitochondrial electron transport chain and prokaryotic electron transport chains are very similar, but no need to me ...
Lecture #10 – 9/26 – Dr. Hirsh
... Form ATP by “burning” NADH through respiration (oxidative phosphorylation) This is a redox reaction with NADH as an intermediate; reduced A (AH2) has a higher energy level than reduced B (BH2) – therefore we have a source of energy for proton pumping. We need to get energy out of he process in small ...
... Form ATP by “burning” NADH through respiration (oxidative phosphorylation) This is a redox reaction with NADH as an intermediate; reduced A (AH2) has a higher energy level than reduced B (BH2) – therefore we have a source of energy for proton pumping. We need to get energy out of he process in small ...
Answers to the RI and UC questions
... 2. Where in a cell does each part of cell respiration take place? Describe how the location of each part of the process is different in bacteria an din more complex cells. The cytosol is the site of glycolysis in both prokaryotic and eukaryotic cells. The mitochondrion is the site of the Krebs cycle ...
... 2. Where in a cell does each part of cell respiration take place? Describe how the location of each part of the process is different in bacteria an din more complex cells. The cytosol is the site of glycolysis in both prokaryotic and eukaryotic cells. The mitochondrion is the site of the Krebs cycle ...
Cellular Respiration - Seattle Central College
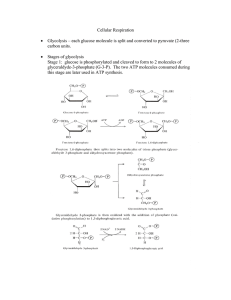
... glucose and the conversion of Fructose-6-phosphate to Fructose-1,6-diphosphate. The net production of ATP per glucose is 2. ...
... glucose and the conversion of Fructose-6-phosphate to Fructose-1,6-diphosphate. The net production of ATP per glucose is 2. ...
Exam Review two KEY
... 4. What happens to the electrons as they move from Photosystem II to Photosystem I? A. Gains energy along the way. B. Energy stays the same. C. Loses energy, this is why a 2nd input of light is needed in Photosystem I. D. Electrons move from Photosystem I to Photosystem II and lose energy along the ...
... 4. What happens to the electrons as they move from Photosystem II to Photosystem I? A. Gains energy along the way. B. Energy stays the same. C. Loses energy, this is why a 2nd input of light is needed in Photosystem I. D. Electrons move from Photosystem I to Photosystem II and lose energy along the ...
Bio 7
... Uses the Citric Acid Cycle, Electron transport system (ETS), ATP-Synthase Takes place in the mitochondria Pyruvate turns into Acetyl-CoA (makes NADH and CO2) Acetyl-CoA enters citric acid cycle more NADH, ATP and CO2 are made NADH are used to power the ETS ETS – pumps H+ ions from matrix to inner me ...
... Uses the Citric Acid Cycle, Electron transport system (ETS), ATP-Synthase Takes place in the mitochondria Pyruvate turns into Acetyl-CoA (makes NADH and CO2) Acetyl-CoA enters citric acid cycle more NADH, ATP and CO2 are made NADH are used to power the ETS ETS – pumps H+ ions from matrix to inner me ...
coupling membrane
... NADH and succinate) in citric acid cycle 4) the oxidation of reduced cofactors by oxygen forming water and releasing energy (respiratory electron transfer) ...
... NADH and succinate) in citric acid cycle 4) the oxidation of reduced cofactors by oxygen forming water and releasing energy (respiratory electron transfer) ...
11.17.11.ATP.synthase
... • ET chain induces electrochemical potential gradient by pumping protons across energy transducing inner mitochondrial membrane against proton and voltage gradient: ...
... • ET chain induces electrochemical potential gradient by pumping protons across energy transducing inner mitochondrial membrane against proton and voltage gradient: ...
Electron Transport System – oxidative phosphorylation
... Some of the steps of glycolysis and the Krebs cycle are redox reactions in which dehydrogenase enzymes transfer electrons from substrates to NAD+, forming NADH. In the third stage of respiration, the electron transport chain accepts electrons from the breakdown products of the first two stages (usua ...
... Some of the steps of glycolysis and the Krebs cycle are redox reactions in which dehydrogenase enzymes transfer electrons from substrates to NAD+, forming NADH. In the third stage of respiration, the electron transport chain accepts electrons from the breakdown products of the first two stages (usua ...
PGS 160-167
... I. If NO OXYGEN is present within the cell (“Anaerobic” means “without oxygen”): A. Fermentation will occur to free up the electron carriers to keep at least Glycolysis going making ATP. 1. Two types of fermentation can occur (It depends on the organism doing it.) (Fig:9.17) a. Alcohol Fermentation ...
... I. If NO OXYGEN is present within the cell (“Anaerobic” means “without oxygen”): A. Fermentation will occur to free up the electron carriers to keep at least Glycolysis going making ATP. 1. Two types of fermentation can occur (It depends on the organism doing it.) (Fig:9.17) a. Alcohol Fermentation ...
Section 9–2 The Krebs Cycle and Electron Transport (pages 226–232)
... 19. What is the energy of the high-energy electrons used for every time 2 high-energy electrons move down the electron transport chain? Their energy is used to transport hydrogen ions across the membrane. ...
... 19. What is the energy of the high-energy electrons used for every time 2 high-energy electrons move down the electron transport chain? Their energy is used to transport hydrogen ions across the membrane. ...
Succinate
... for NAD+ (-0.219 vs. -0.315 V). This means that NADH has a more positive oxidation potential and hence is a better reducing agent than FADH2, thus yields more energy when it coughs up its electrons, resulting in greater ATP production. Types of electron transport components: - Cytochromes contain he ...
... for NAD+ (-0.219 vs. -0.315 V). This means that NADH has a more positive oxidation potential and hence is a better reducing agent than FADH2, thus yields more energy when it coughs up its electrons, resulting in greater ATP production. Types of electron transport components: - Cytochromes contain he ...
Cellular Respiration
... In matrix of mitochondria, needs mitochondria does not require oxygen Splits C-C bonds ( in acetyl ) Generates some ATP, lots of NADH and FADH2 and CO2 as waste ...
... In matrix of mitochondria, needs mitochondria does not require oxygen Splits C-C bonds ( in acetyl ) Generates some ATP, lots of NADH and FADH2 and CO2 as waste ...
Lecture 15
... - Krebs Cycle takes place within the mitochondrial matrix, and breaks a pyruvate into CO2 and produce some ATP and NADH. - Some steps of Glycolysis and Krebs Cycle are Redox in which dehydrogenase enzyme reduces NAD+ into NADH. - Some of ATP is produced at these two steps via (substrate-levelphospho ...
... - Krebs Cycle takes place within the mitochondrial matrix, and breaks a pyruvate into CO2 and produce some ATP and NADH. - Some steps of Glycolysis and Krebs Cycle are Redox in which dehydrogenase enzyme reduces NAD+ into NADH. - Some of ATP is produced at these two steps via (substrate-levelphospho ...
9.1 Catabolic pathways yield energy by oxidizing organic fuels
... Feedback Inhibition controls the anabolic and catabolic pathways. •Phosphofructokinase- pace maker of respiration. It is an allosteric enzymes with Receptor sites fro AMP and ATP. •ATP binds to Phospho. and respiration is inhibited. •AMP binds to Phospo. and the respiration is stimulated. •Cells are ...
... Feedback Inhibition controls the anabolic and catabolic pathways. •Phosphofructokinase- pace maker of respiration. It is an allosteric enzymes with Receptor sites fro AMP and ATP. •ATP binds to Phospho. and respiration is inhibited. •AMP binds to Phospo. and the respiration is stimulated. •Cells are ...
CHE 4310 Fall 2011
... the breakdown of fructose, lactose, or sucrose are defective. However, there are very few cases of people having a genetic disease in which one of the enzymes of glycolysis is severely affected. Why do you suppose such mutations are seen so rarely? ...
... the breakdown of fructose, lactose, or sucrose are defective. However, there are very few cases of people having a genetic disease in which one of the enzymes of glycolysis is severely affected. Why do you suppose such mutations are seen so rarely? ...
Harvesting Chemical Energy
... Uncouplers, like dinitrophenol (DNP), cause cristae to leak H+, cannot maintain H+ gradient ...
... Uncouplers, like dinitrophenol (DNP), cause cristae to leak H+, cannot maintain H+ gradient ...
A closer look at cellular respiration
... produces a small amount of ATP. Most of the energy of the original glucose molecule, however, remains in the two pyruvate molecules. Glycolysis may be followed by a pathway that does not require ...
... produces a small amount of ATP. Most of the energy of the original glucose molecule, however, remains in the two pyruvate molecules. Glycolysis may be followed by a pathway that does not require ...
Quiz 2: Bio 160 Saunders
... MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following is a function of the plasma membrane? A) control center of the cell B) protein synthesis C) fat synthesis D) intracellular digestion E) regulation of the passage of materi ...
... MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following is a function of the plasma membrane? A) control center of the cell B) protein synthesis C) fat synthesis D) intracellular digestion E) regulation of the passage of materi ...
Cellular Respiration
... oxidative reduction & the kreb’s cycle. • Possible for 36 ATP to be made. ...
... oxidative reduction & the kreb’s cycle. • Possible for 36 ATP to be made. ...
Name CELLULAR RESPIRATION Let`s take a look back
... – Produces burning feeling in muscle cells – Occurs when body is worked to the point that more oxygen is being used than taken in – Produces __________________________________________________________ ...
... – Produces burning feeling in muscle cells – Occurs when body is worked to the point that more oxygen is being used than taken in – Produces __________________________________________________________ ...
Practice Test Questions
... it donates H's and electrons oxygen combines with carbon from glucose to form CO2 it transfers H's from the Krebs cycle by temporarily ...
... it donates H's and electrons oxygen combines with carbon from glucose to form CO2 it transfers H's from the Krebs cycle by temporarily ...
Oxidative phosphorylation
Oxidative phosphorylation (or OXPHOS in short) is the metabolic pathway in which the mitochondria in cells use their structure, enzymes, and energy released by the oxidation of nutrients to reform ATP. Although the many forms of life on earth use a range of different nutrients, ATP is the molecule that supplies energy to metabolism. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis.During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cells' intermembrane space. These linked sets of proteins are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme called ATP synthase; this process is known as chemiosmosis. This enzyme uses this energy to generate ATP from adenosine diphosphate (ADP), in a phosphorylation reaction. This reaction is driven by the proton flow, which forces the rotation of a part of the enzyme; the ATP synthase is a rotary mechanical motor.Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging (senescence). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.