presentation source
... A typical nucleus has a radius of about 10-15 meters, yet Rutherford was able to “see” the nucleus of a gold atom. How did he do it? We usually see by detecting light that has bounced off objects into our eyes. If the object is very small compared to the wavelength of the light, then the light diffr ...
... A typical nucleus has a radius of about 10-15 meters, yet Rutherford was able to “see” the nucleus of a gold atom. How did he do it? We usually see by detecting light that has bounced off objects into our eyes. If the object is very small compared to the wavelength of the light, then the light diffr ...
Chapter 5 Electrons In Atoms 5.1 Models of the Atom The
... The number of electron allowed in each of the first four energy levels is shown in table 5.2 5.2 Electron Arrangement in Atoms Electron Configurations The ways in which ___________________ are arranged in various orbitals around the nuclei of atoms are called electron configurations. Three rules - t ...
... The number of electron allowed in each of the first four energy levels is shown in table 5.2 5.2 Electron Arrangement in Atoms Electron Configurations The ways in which ___________________ are arranged in various orbitals around the nuclei of atoms are called electron configurations. Three rules - t ...
section on Compton effect
... Compton verified his result experimentally using the characteristic x-ray line of wavelength 0.0711 nm from molybdenum for the incident monochromatic photons and scattering these photons from electrons in graphite. The wavelength of the scattered photons was measured using a Bragg crystal spectromet ...
... Compton verified his result experimentally using the characteristic x-ray line of wavelength 0.0711 nm from molybdenum for the incident monochromatic photons and scattering these photons from electrons in graphite. The wavelength of the scattered photons was measured using a Bragg crystal spectromet ...
Constituents and Shapes of Nuclei and Nucleons
... The kinetic energy of protons in the nucleus is about 1 million times larger than the kinetic energy of electrons in an atom, just by the Heisenberg uncertainty principle, and in good agreement with experimental data. The high energy of nuclear processes is an inevitable consequence of the small siz ...
... The kinetic energy of protons in the nucleus is about 1 million times larger than the kinetic energy of electrons in an atom, just by the Heisenberg uncertainty principle, and in good agreement with experimental data. The high energy of nuclear processes is an inevitable consequence of the small siz ...
ppt - UCSC Bayesian Data Analysis Workshop
... – use ideas from sequential importance sampling to only generate runs that with high probability trigger the detectors that fired – non-parametric representation of the distribution that allows for tracking of multiple hypotheses ...
... – use ideas from sequential importance sampling to only generate runs that with high probability trigger the detectors that fired – non-parametric representation of the distribution that allows for tracking of multiple hypotheses ...
Quantum Mechanics
... We know some small particles like electrons, protons, and neutrons. Quantum physics even describes the particles which make these particles! (The model of an atom that you were taught in high-school is a approximation). The electrons don't orbit like planets; they form blurred clouds of probabiliti ...
... We know some small particles like electrons, protons, and neutrons. Quantum physics even describes the particles which make these particles! (The model of an atom that you were taught in high-school is a approximation). The electrons don't orbit like planets; they form blurred clouds of probabiliti ...
Atomic (proton) number = is the number of protons found in the
... biological process. Effective half-life = combination of physical and biological half lives Annihilation = interaction between an elementary particle and its antiparticle resulting in their disappearance and emission of different particles Linear energy transfer (LET) = quotient of dE by dI, for a m ...
... biological process. Effective half-life = combination of physical and biological half lives Annihilation = interaction between an elementary particle and its antiparticle resulting in their disappearance and emission of different particles Linear energy transfer (LET) = quotient of dE by dI, for a m ...
What`s common these things
... All ordinary matter belongs to this group 1st family These particles existed right after the Big Bang. Nowadays they are found in cosmic rays and they are produced at the accelerator experiments 2nd family 3rd family Strong interaction It has the fastest messengers on the short distance, the gluons ...
... All ordinary matter belongs to this group 1st family These particles existed right after the Big Bang. Nowadays they are found in cosmic rays and they are produced at the accelerator experiments 2nd family 3rd family Strong interaction It has the fastest messengers on the short distance, the gluons ...
Chemistry – Ch 4 Review Sheet
... ___1. Given a particle of mass m and velocity v, de Broglie’s hypothesis allows you to predict the ___ a. position of the particle c. wavelength of the particle b. diameter of the particle d. charge of the particle ___2. Which of the following is a possible value of electron spin? a. 0 b. +1/2 c. 1 ...
... ___1. Given a particle of mass m and velocity v, de Broglie’s hypothesis allows you to predict the ___ a. position of the particle c. wavelength of the particle b. diameter of the particle d. charge of the particle ___2. Which of the following is a possible value of electron spin? a. 0 b. +1/2 c. 1 ...
Chemistry – Ch 4 Review Sheet
... Chemistry – Ch 5 Review Sheet C 1. Given a particle of mass m and velocity v, de Broglie’s hypothesis allows you to predict the ___ a. position of the particle c. wavelength of the particle b. diameter of the particle d. charge of the particle B 2. Which of the following is a possible value of elect ...
... Chemistry – Ch 5 Review Sheet C 1. Given a particle of mass m and velocity v, de Broglie’s hypothesis allows you to predict the ___ a. position of the particle c. wavelength of the particle b. diameter of the particle d. charge of the particle B 2. Which of the following is a possible value of elect ...
Higher Physics Content Statements
... The Bohr model of the atom. Electrons can be excited to higher energy levels by an input of energy. Ionisation level is the level at which an electron is free from the atom. Zero potential energy is defined as equal to that of the ionisation level, implying that other energy levels have negative val ...
... The Bohr model of the atom. Electrons can be excited to higher energy levels by an input of energy. Ionisation level is the level at which an electron is free from the atom. Zero potential energy is defined as equal to that of the ionisation level, implying that other energy levels have negative val ...
subatomic particle
... radioactive decays would not conserve energy or momentum. • The 2002 Physics Nobel prize to Davis & Koshiba was for detecting neutrinos emitted by fusion in our sun. ...
... radioactive decays would not conserve energy or momentum. • The 2002 Physics Nobel prize to Davis & Koshiba was for detecting neutrinos emitted by fusion in our sun. ...
1 eV - Nikhef
... * The bunching cavities 2 regulate the speed of the electrons so that they arrive in bunches at the output cavity. * The bunches of electrons excite microwaves in the output cavity 3 of the klystron. * The microwaves flow into the waveguide 4, which transports them to the accelerator. * The electron ...
... * The bunching cavities 2 regulate the speed of the electrons so that they arrive in bunches at the output cavity. * The bunches of electrons excite microwaves in the output cavity 3 of the klystron. * The microwaves flow into the waveguide 4, which transports them to the accelerator. * The electron ...
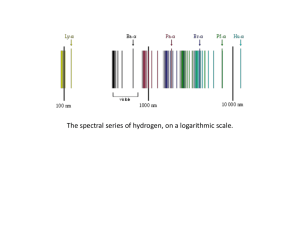
Rutherford–Bohr model
... atomic nucleus and where an electron jump between orbits is accompanied by an emitted or absorbed amount of electromagnetic energy (hν).[1] The orbits in which the electron may travel are shown as grey circles; their radius increases as n2, where n is the principal quantum number. The 3 → 2 transiti ...
... atomic nucleus and where an electron jump between orbits is accompanied by an emitted or absorbed amount of electromagnetic energy (hν).[1] The orbits in which the electron may travel are shown as grey circles; their radius increases as n2, where n is the principal quantum number. The 3 → 2 transiti ...
3. THE DEGENERATE ELECTRON GAS Chapter 1 : SECOND QUANTIZATION
... because there will be intermediate results that are singular in the limit. The singularities will cancel before we take the limit. The thermodynamic limit. This is the limit N → ∞, V → ∞, with n = N/V constant and finite. As we go along we’ll make approximations that are valid in this limit. ...
... because there will be intermediate results that are singular in the limit. The singularities will cancel before we take the limit. The thermodynamic limit. This is the limit N → ∞, V → ∞, with n = N/V constant and finite. As we go along we’ll make approximations that are valid in this limit. ...
32 The Atom and the Quantum Answers and Solutions for Chapter
... 1. Most particles remain undeflected because of the empty space within atoms. 2. Rutherford discovered the atomic nucleus, that it was tiny, positive, and massive. 3. Franklin postulated that electricity is an electric fluid that flows from place to place. 4. A cathode ray is a beam of electrons. 5. ...
... 1. Most particles remain undeflected because of the empty space within atoms. 2. Rutherford discovered the atomic nucleus, that it was tiny, positive, and massive. 3. Franklin postulated that electricity is an electric fluid that flows from place to place. 4. A cathode ray is a beam of electrons. 5. ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.