Ch. 6 notes
... Calculate the energy required to excite the hydrogen electron from level n=1 to level n=2. Also calculate the wavelength of light that must be absorbed by a hydrogen atom in its ground state to reach this excited state. ...
... Calculate the energy required to excite the hydrogen electron from level n=1 to level n=2. Also calculate the wavelength of light that must be absorbed by a hydrogen atom in its ground state to reach this excited state. ...
4.1 and 4.2 notes.pptx
... ____________________________in simple whole-number ratios to form compounds (2H to 1O make water) 4. Chemical reactions occur when atoms _________________________from each other, ____________________, or _________________________in a different combination. Atoms of one element, however, are ________ ...
... ____________________________in simple whole-number ratios to form compounds (2H to 1O make water) 4. Chemical reactions occur when atoms _________________________from each other, ____________________, or _________________________in a different combination. Atoms of one element, however, are ________ ...
File
... h is Planck’s constant h= 6.63 x 10-34 J.s u is equal to the frequency of light. u = f = frequency ...
... h is Planck’s constant h= 6.63 x 10-34 J.s u is equal to the frequency of light. u = f = frequency ...
Chapter 7
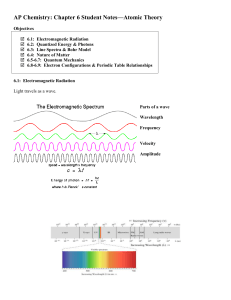
... A. Wave Nature of light Light is electromagnetic radiation. A type of energy embodies in oscillating electric and magnetic fields ...
... A. Wave Nature of light Light is electromagnetic radiation. A type of energy embodies in oscillating electric and magnetic fields ...
Chapter 14
... b. anode c. atomic number d. beta particle e. cathode f. electron g. actinides h. electron cloud i. element j. half-life k. isotope l. mass number m. neutron n. proton o. radioactive decay p. transition q. transmutation ...
... b. anode c. atomic number d. beta particle e. cathode f. electron g. actinides h. electron cloud i. element j. half-life k. isotope l. mass number m. neutron n. proton o. radioactive decay p. transition q. transmutation ...
Motion Along a Straight Line at Constant
... which emits electrons, a nearby positive anode attracts these electrons which pass through a hole in the anode to form a beam. This is called Thermionic emission. The potential difference between the anode and cathode controls the speed of the electrons. ...
... which emits electrons, a nearby positive anode attracts these electrons which pass through a hole in the anode to form a beam. This is called Thermionic emission. The potential difference between the anode and cathode controls the speed of the electrons. ...
Slide 1
... The end of classical physics: photons, electrons, atoms Last time: Discovery of the electron: Charge quantization photo-electric effect: light behaves as a particle Discovery of the nucleus: Rutherford scattering experiment Today: Atomic spectra: emission and absorption. Evidence for atomic ener ...
... The end of classical physics: photons, electrons, atoms Last time: Discovery of the electron: Charge quantization photo-electric effect: light behaves as a particle Discovery of the nucleus: Rutherford scattering experiment Today: Atomic spectra: emission and absorption. Evidence for atomic ener ...
Force on a Charged Particle
... Force on a Charged Particle • Charged particles are not confined to a wire. • Cathode-ray tube in monitors and TVs use magnetic fields to deflect electrons to form pictures on the screen. • The screen in coated with phosphor to glow when an electron hits it. ...
... Force on a Charged Particle • Charged particles are not confined to a wire. • Cathode-ray tube in monitors and TVs use magnetic fields to deflect electrons to form pictures on the screen. • The screen in coated with phosphor to glow when an electron hits it. ...
The Chemical Earth (8.2.3)
... • As we move away from the nucleus into higher energy levels, nuclear attraction becomes less. (See “Atomic Size” power point file with this unit) ...
... • As we move away from the nucleus into higher energy levels, nuclear attraction becomes less. (See “Atomic Size” power point file with this unit) ...
ECE692 Slides 3: Solid State Physics (Updated 09/18 - UTK-EECS
... Block electron ħk is the crystal momentum, which is not a momentum, but is treated as momentum in the semiclassical theory. n is the band index. 2 | k k 0 |2 E (k ) En(k) = En(k+K) ...
... Block electron ħk is the crystal momentum, which is not a momentum, but is treated as momentum in the semiclassical theory. n is the band index. 2 | k k 0 |2 E (k ) En(k) = En(k+K) ...
Slide 1
... measurements were known early on in our saga. • The need for a strong, short range (strong) force was obvious. • The quantum theory of the electromagnetic field (QED) involved photons and was a phenomenal success. • Yukawa generalized QED to the nuclear force and predicted a mass about the same as t ...
... measurements were known early on in our saga. • The need for a strong, short range (strong) force was obvious. • The quantum theory of the electromagnetic field (QED) involved photons and was a phenomenal success. • Yukawa generalized QED to the nuclear force and predicted a mass about the same as t ...
Quantum Theory Historical Reference
... 12. Louis de Broglie(1892-1987): Predicted that fundamental particles might display wave properties under certain circumstances. His doctoral thesis (1925) proposed that a particle of mass (m) and velocity (v) should have a wavelength associated with it. Ultimately explains the quantized energy of ...
... 12. Louis de Broglie(1892-1987): Predicted that fundamental particles might display wave properties under certain circumstances. His doctoral thesis (1925) proposed that a particle of mass (m) and velocity (v) should have a wavelength associated with it. Ultimately explains the quantized energy of ...
From electrons to quarks – the development of Particle Physics
... electrons liberated from atom or molecule, can be collected, and charge is detected collisions Particles moving through an electric field are accelerated (decelerated) Particles moving through a magnetic field are bent ...
... electrons liberated from atom or molecule, can be collected, and charge is detected collisions Particles moving through an electric field are accelerated (decelerated) Particles moving through a magnetic field are bent ...
Atom 1 - UF Physics
... verified experimentally by Geiger and Marsden in 1913. Thus, the Rutherford nucleus model is correct! Since no deviation was observed from the predicted scattering rate, one can derive an upper limit on the size of the nucleus by calculating the distance of closest approach for a head-on collision. ...
... verified experimentally by Geiger and Marsden in 1913. Thus, the Rutherford nucleus model is correct! Since no deviation was observed from the predicted scattering rate, one can derive an upper limit on the size of the nucleus by calculating the distance of closest approach for a head-on collision. ...
Microscopic Theory of Conduction
... Other problems with the classical model... • The “electron gas” should produce a much higher heat capacity than is observed • Classical Model has no way to explain phenomena such as Contact Potentials or Superconductivity ...
... Other problems with the classical model... • The “electron gas” should produce a much higher heat capacity than is observed • Classical Model has no way to explain phenomena such as Contact Potentials or Superconductivity ...
The Quantum Mechanical Behavior of Light and Matter
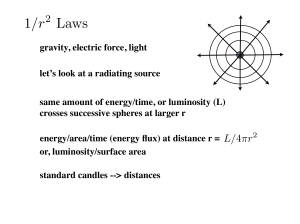
... energy/area/time (energy flux) at distance r = L/4πr or, luminosity/surface area standard candles --> distances ...
... energy/area/time (energy flux) at distance r = L/4πr or, luminosity/surface area standard candles --> distances ...
06-Nuclear shorter
... High energy e-m wave (A Photon) No charge - not deflected by field Very penetrating – Need lead to stop most of them Not very ionizing Release energy after reaction ...
... High energy e-m wave (A Photon) No charge - not deflected by field Very penetrating – Need lead to stop most of them Not very ionizing Release energy after reaction ...
2·QUIZLET VOCABULARY: Quantum Numbers Study online at
... 3. electron configuration: the arrangement of electrons around nucleus in an atom 4. Hunds rule: orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin 5. Magnetic (orbital) ...
... 3. electron configuration: the arrangement of electrons around nucleus in an atom 4. Hunds rule: orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin 5. Magnetic (orbital) ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.