Bohr Atom
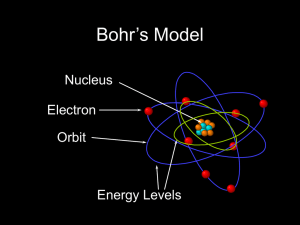
... The Bohr model of the atom, like many ideas in the history of science, was at first prompted by and later partially disproved by experimentation. http://en.wikipedia.org/wiki/Category:Chemistry ...
... The Bohr model of the atom, like many ideas in the history of science, was at first prompted by and later partially disproved by experimentation. http://en.wikipedia.org/wiki/Category:Chemistry ...
Energy Loss - High Energy Physics at Notre Dame
... Tmax is often approximated by 2me22. re is the classical electron radius (re = e2 / mec2 = 2.82 x10-13 cm) (radius of a classical distribution of the electron charge with electrostatic self-energy equal to the electron mass). I is the mean ionization energy. ...
... Tmax is often approximated by 2me22. re is the classical electron radius (re = e2 / mec2 = 2.82 x10-13 cm) (radius of a classical distribution of the electron charge with electrostatic self-energy equal to the electron mass). I is the mean ionization energy. ...
Relativity
... 1- In photoelectric effect, the incident photon energy is 3.6 eV. If the work function of the material is 1.56 eV, Calculate the electron stopping potential. 2- If the work function for a given material is 2.56 eV, calculate the threshold frequency and the threshold wavelength. 3- The minimum wavele ...
... 1- In photoelectric effect, the incident photon energy is 3.6 eV. If the work function of the material is 1.56 eV, Calculate the electron stopping potential. 2- If the work function for a given material is 2.56 eV, calculate the threshold frequency and the threshold wavelength. 3- The minimum wavele ...
Non-KAM dynamical chaos in semiconductor superlattices Arkadii Krokhin, UNT
... I will present our new results concerning electron dynamics in semiconductor superlattices in the presence of non-parallel electric and magnetic field. In this geometry the electrons in the superlattice miniband turn out to form a non-KAM dynamical system that exhibits a non-traditional chaotic beha ...
... I will present our new results concerning electron dynamics in semiconductor superlattices in the presence of non-parallel electric and magnetic field. In this geometry the electrons in the superlattice miniband turn out to form a non-KAM dynamical system that exhibits a non-traditional chaotic beha ...
Assignment #1
... Modern Physics 3rd Ed. Serway, Moses, and Moyer- Chapter 1 #12, 20, 26, 27, 28, 31 Problem #7: A relativistic subatomic particle of mass m is moving away from the detector when it spontaneously decays, sending a photon toward the detector. The photon is observed to be red shifted by a factor of 100, ...
... Modern Physics 3rd Ed. Serway, Moses, and Moyer- Chapter 1 #12, 20, 26, 27, 28, 31 Problem #7: A relativistic subatomic particle of mass m is moving away from the detector when it spontaneously decays, sending a photon toward the detector. The photon is observed to be red shifted by a factor of 100, ...
THINGSYOUNEEDTOKNOW-modern
... CONTINUOUS SPECTRUM-A smooth spectrum of all colors of the rainbow. Generated by light bulb ABSORPTION SPECTRUM-looks like continuous spectrum with some spectral lines absorbed By a cool gas EMISSION SPECTRUM-a spectrum made up of only a few spectral lines emitted by an excited gas. Energy of photon ...
... CONTINUOUS SPECTRUM-A smooth spectrum of all colors of the rainbow. Generated by light bulb ABSORPTION SPECTRUM-looks like continuous spectrum with some spectral lines absorbed By a cool gas EMISSION SPECTRUM-a spectrum made up of only a few spectral lines emitted by an excited gas. Energy of photon ...
FRCRIII - hullrad Radiation Physics
... Probability of a photoelectric interaction decreases with increasing photon energy (E) increases with atomic number (Z) ...
... Probability of a photoelectric interaction decreases with increasing photon energy (E) increases with atomic number (Z) ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.