
Chapter 27
... A continuous beam of X-rays is incident on the crystal The diffracted radiation is very intense in certain directions ...
... A continuous beam of X-rays is incident on the crystal The diffracted radiation is very intense in certain directions ...
FREE ELECTRON THEORY
... crystal. A particle-like entity can be obtained by superposing the plane wave states to form a wavepacket. ...
... crystal. A particle-like entity can be obtained by superposing the plane wave states to form a wavepacket. ...
particlephysics
... baryon and lepton number e.g. n & p have baryon number = 1 n & p have baryon number = -1 ...
... baryon and lepton number e.g. n & p have baryon number = 1 n & p have baryon number = -1 ...
IPS Unit 8 – Periodic Table Structure of the Atom Worksheet
... 3. Are electrons, protons, or neutrons the smallest particles? If not, what are? ...
... 3. Are electrons, protons, or neutrons the smallest particles? If not, what are? ...
Chapter 2 Study Guide
... 1. The first subatomic particle discovered was ___________________________. 2. The only subatomic particle that does not carry an electric charge is the __________. 3. The atomic number of an element whose atoms have 12 protons and 11 neutrons is _____. 4. The mass number of an element whose atoms h ...
... 1. The first subatomic particle discovered was ___________________________. 2. The only subatomic particle that does not carry an electric charge is the __________. 3. The atomic number of an element whose atoms have 12 protons and 11 neutrons is _____. 4. The mass number of an element whose atoms h ...
2.1.7 particle movement in magnetic fields
... can be to change speed or direction. The effect of a magnetic field on a charged particle can only be to change its direction. This is because the force applied is always perpendicular to its motion. ...
... can be to change speed or direction. The effect of a magnetic field on a charged particle can only be to change its direction. This is because the force applied is always perpendicular to its motion. ...
19.1 Reinforcement WKT to project
... 3. Are electrons, protons, or neutrons the smallest particles? If not, what are? 4. How many types of quarks are there and what is the name of one of them? 5. Why do scientists use models to study atoms? 6. Why has the atomic model changed over time? 7. Why is the current atomic model called the “El ...
... 3. Are electrons, protons, or neutrons the smallest particles? If not, what are? 4. How many types of quarks are there and what is the name of one of them? 5. Why do scientists use models to study atoms? 6. Why has the atomic model changed over time? 7. Why is the current atomic model called the “El ...
Phys 209BH
... The maximum kinetic energy of the photoelectrons released from a metal surface when exposed to 492 nm light is 9.9 10-20 J. Energy drops to 3.8 10-20 J if the wavelength is changed to 579 nm. Use this data to calculate plank’s constant h and work function . ...
... The maximum kinetic energy of the photoelectrons released from a metal surface when exposed to 492 nm light is 9.9 10-20 J. Energy drops to 3.8 10-20 J if the wavelength is changed to 579 nm. Use this data to calculate plank’s constant h and work function . ...
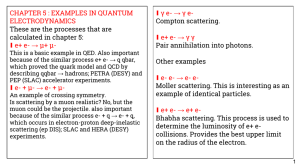
CHAPTER 5 : EXAMPLES IN QUANTUM γ e- → γ e- ∎ ELECTRODYNAMICS
... These were important experiments in the history of high-energy physics. From the particle data group, the figure shows the ratio R = σ ( e e → hadrons ) / σ ( e ebar → μ μbar) . The underlying process in hadron production is e- + e+ → q + qbar. Neglecting QCD interactions we would just have R = cons ...
... These were important experiments in the history of high-energy physics. From the particle data group, the figure shows the ratio R = σ ( e e → hadrons ) / σ ( e ebar → μ μbar) . The underlying process in hadron production is e- + e+ → q + qbar. Neglecting QCD interactions we would just have R = cons ...
PPT - Lawless Teaching : Home
... Millikan found the charge of an electron by suspending charged oil drops in an electric field. The electric force required was equal to the weight of the drops. He determined the charge to be either 1.6 × 10−19 C or some integer multiple of this. He concluded that this was the charge on the ...
... Millikan found the charge of an electron by suspending charged oil drops in an electric field. The electric force required was equal to the weight of the drops. He determined the charge to be either 1.6 × 10−19 C or some integer multiple of this. He concluded that this was the charge on the ...
Test Paper No. 12 (Physics)
... of an electron in an atom? 6. Define distance of closest approach and derive an expression for it. ...
... of an electron in an atom? 6. Define distance of closest approach and derive an expression for it. ...
Charges in a Magnetic Field
... If the charge on the particle is known, the mass can be calculated. ...
... If the charge on the particle is known, the mass can be calculated. ...
Wave as particle 2
... When photon with energy above the rest mass of two electrons ( 2me c 2 ) interact with the electric field of a nucleus, this photon may be turned into a pair of electron and positron. This process is called pair production through which energy gets turned into mass. Positron is the anti-particle of ...
... When photon with energy above the rest mass of two electrons ( 2me c 2 ) interact with the electric field of a nucleus, this photon may be turned into a pair of electron and positron. This process is called pair production through which energy gets turned into mass. Positron is the anti-particle of ...
elementary particles history
... radioactive decay series, proposed atomic transmutation in radioactive elements (1902), showed a particles are He nuclei, developed nuclear model of atom (1910) based on a particle scattering, demonstrated artificial transmutation (proton ejected when a collided with N nucleus, measured nuclear size ...
... radioactive decay series, proposed atomic transmutation in radioactive elements (1902), showed a particles are He nuclei, developed nuclear model of atom (1910) based on a particle scattering, demonstrated artificial transmutation (proton ejected when a collided with N nucleus, measured nuclear size ...
Particle acceleration by electric field in an 3D RCS
... General analysis of the particle motion: trajectories Electric field is the force that governs a straightforward movement of ...
... General analysis of the particle motion: trajectories Electric field is the force that governs a straightforward movement of ...
Historical Introduction to the Elementary Particles
... • Unfortunately, the next heavier atom (helium), although it does indeed carry two electrons, weighs four times as much as hydrogen, and lithium (three electrons) is seven times the weight of hydrogen, and so it goes. This dilemma was finally resolved in 1932 with Chadwick‘s discovery of the neutro ...
... • Unfortunately, the next heavier atom (helium), although it does indeed carry two electrons, weighs four times as much as hydrogen, and lithium (three electrons) is seven times the weight of hydrogen, and so it goes. This dilemma was finally resolved in 1932 with Chadwick‘s discovery of the neutro ...
introduction [Kompatibilitätsmodus]
... Millikan‘s experiment (1911, Nobel Price in 1923): Oil droplets are sprayed in a chamber between two electrodes and charged by ionizing radiation. Their motion is observed by a microscope. ...
... Millikan‘s experiment (1911, Nobel Price in 1923): Oil droplets are sprayed in a chamber between two electrodes and charged by ionizing radiation. Their motion is observed by a microscope. ...
Physics 105 - Multiple Choice Questions Ch 16
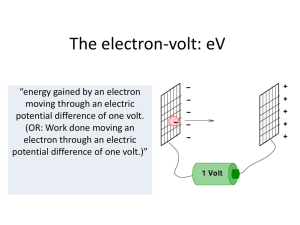
... move an electron 1 meter C) the energy gained by an electron in moving through a potential difference of 1 volt D) the energy needed to move an electron through 1 meter in any electric field E) the work done when 1 coulomb of charge is moved through a potential difference of 1 volt ...
... move an electron 1 meter C) the energy gained by an electron in moving through a potential difference of 1 volt D) the energy needed to move an electron through 1 meter in any electric field E) the work done when 1 coulomb of charge is moved through a potential difference of 1 volt ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.


















![introduction [Kompatibilitätsmodus]](http://s1.studyres.com/store/data/017596641_1-03cad833ad630350a78c42d7d7aa10e3-300x300.png)
