OBJECTIVE WORKSHEET Quantum Theory 1. How did
... 3. How many energy levels for electrons does the chapter discuss? 4. Who discovered the QUANTUM MECHANICAL MODEL? 5. What shape does the s and p orbitals have? 6. What does "n" stand for when we discuss atomic orbitals? 7. What is the maximum number of electrons allowed in when n=4? 8. What is "neon ...
... 3. How many energy levels for electrons does the chapter discuss? 4. Who discovered the QUANTUM MECHANICAL MODEL? 5. What shape does the s and p orbitals have? 6. What does "n" stand for when we discuss atomic orbitals? 7. What is the maximum number of electrons allowed in when n=4? 8. What is "neon ...
03 Homework File
... To be handed in: Tuesday 31st January Answer scripts should be returned in the box provided on the first floor in the Physics Building (G.O. Jones) by 4pm on the day quoted above. Course title, week number and student’s name should appear on every sheet of the worked exercises, which should be secur ...
... To be handed in: Tuesday 31st January Answer scripts should be returned in the box provided on the first floor in the Physics Building (G.O. Jones) by 4pm on the day quoted above. Course title, week number and student’s name should appear on every sheet of the worked exercises, which should be secur ...
The Compton Effect, or Compton scattering – conclusive evidence
... than the original x-ray photons. The scattered x-rays had lost energy. Where did the extra energy go? The energy lost by the x-ray photons, as evidenced by the photons’ increased wavelength, increases the kinetic energy of the scattered electrons. Sound like billiards? It should! The collision is in ...
... than the original x-ray photons. The scattered x-rays had lost energy. Where did the extra energy go? The energy lost by the x-ray photons, as evidenced by the photons’ increased wavelength, increases the kinetic energy of the scattered electrons. Sound like billiards? It should! The collision is in ...
Ideas of Modern Physics
... Which has the longest wavelength? a. beta particle. b. gamma ray. c. alpha particle. d. all the same. e. depends on gamma ray energy. 3. Particular red (600 nm) and blue (300 nm) lasers both produce 10 mW of power. How do the number of photons per second from each compare? a. Both the same. b. Blue ...
... Which has the longest wavelength? a. beta particle. b. gamma ray. c. alpha particle. d. all the same. e. depends on gamma ray energy. 3. Particular red (600 nm) and blue (300 nm) lasers both produce 10 mW of power. How do the number of photons per second from each compare? a. Both the same. b. Blue ...
Physics 107: Ideas of Modern Physics
... Which has the longest wavelength? a. beta particle. b. gamma ray. c. alpha particle. d. all the same. e. depends on gamma ray energy. 3. Particular red (600 nm) and blue (300 nm) lasers both produce 10 mW of power. How do the number of photons per second from each compare? a. Both the same. b. Blue ...
... Which has the longest wavelength? a. beta particle. b. gamma ray. c. alpha particle. d. all the same. e. depends on gamma ray energy. 3. Particular red (600 nm) and blue (300 nm) lasers both produce 10 mW of power. How do the number of photons per second from each compare? a. Both the same. b. Blue ...
Lesson 1 - Tarleton State University
... wave but interacts as a particle as claimed by Einstein then particles must also have wave properties! Furthermore, the basic equations must be analogous since all particles are waves and vice-versa. ...
... wave but interacts as a particle as claimed by Einstein then particles must also have wave properties! Furthermore, the basic equations must be analogous since all particles are waves and vice-versa. ...
Suspended Nanomaterials - Facility for Light Scattering
... – Described Brownian motion based on Kinetic theory of heat ...
... – Described Brownian motion based on Kinetic theory of heat ...
How Are Electric And Magnetic Fields Used To Steer
... Force on a charged particle in a magnetic field equation: F = B q v sin θ F = force (N) B = magnetic field strength (T) q = charge on the particle (C) v = velocity of the particle (m/s) (Angle θ is between the direction of the beam and the magnetic field direction) ...
... Force on a charged particle in a magnetic field equation: F = B q v sin θ F = force (N) B = magnetic field strength (T) q = charge on the particle (C) v = velocity of the particle (m/s) (Angle θ is between the direction of the beam and the magnetic field direction) ...
Introduction to Quantum Mechanics AEP3610 Professor Scott
... • electron energies En = – Z2 E0 n– 2 and that is very good! • they crowd closer and closer together and there are an infinite number of them ionization at zero energy • the speeds get smaller as n goes up ~ n– 1… that’s sort of OK • the radii get larger as n goes up ~ n2… that’s sort of not so OK ...
... • electron energies En = – Z2 E0 n– 2 and that is very good! • they crowd closer and closer together and there are an infinite number of them ionization at zero energy • the speeds get smaller as n goes up ~ n– 1… that’s sort of OK • the radii get larger as n goes up ~ n2… that’s sort of not so OK ...
Key Terms alpha particle - A positively charged particle
... fission - A nuclear reaction in which an atomic nucleus, especially a heavy nucleus such as an isotope of uranium, splits into fragments, usually two fragments of comparable mass, releasing from 100 million to several hundred million electron volts of energy. ...
... fission - A nuclear reaction in which an atomic nucleus, especially a heavy nucleus such as an isotope of uranium, splits into fragments, usually two fragments of comparable mass, releasing from 100 million to several hundred million electron volts of energy. ...
Historic Development by Mihai
... wavelength is short enough. The photoelectric effect is observed below some threshold wavelength which is specific to the material. Especially the fact that light of large wavelengths has no effect at all even if it is extremely intensive, appeared mysterious for the scientists. Albert Einstein fina ...
... wavelength is short enough. The photoelectric effect is observed below some threshold wavelength which is specific to the material. Especially the fact that light of large wavelengths has no effect at all even if it is extremely intensive, appeared mysterious for the scientists. Albert Einstein fina ...
Physics 107 Exam #1 September 12, 1994 Your name: Multiple
... 1. Find the change in frequency of a photon of red light whose original frequency was 7.3x1014 Hz when it falls through 100 m just above the surface of the earth. (a) 4.80x10 -19 Hz, (b) 15.25x1014 Hz, (c) 1.80 Hz, (d) 7.95 Hz. 2. A meter stick appears only 60 cm long to an observer. How long does i ...
... 1. Find the change in frequency of a photon of red light whose original frequency was 7.3x1014 Hz when it falls through 100 m just above the surface of the earth. (a) 4.80x10 -19 Hz, (b) 15.25x1014 Hz, (c) 1.80 Hz, (d) 7.95 Hz. 2. A meter stick appears only 60 cm long to an observer. How long does i ...
Misc. Ch 27 Topics
... • Scattered x-rays had a slightly longer wavelength than incident • Energies of scattered rays were lower • Energy reduction depended on the angle scattered ...
... • Scattered x-rays had a slightly longer wavelength than incident • Energies of scattered rays were lower • Energy reduction depended on the angle scattered ...
Cyclotron - schoolphysics
... It is interesting to consider the frequency of the accelerating voltage as the energy of the particles gets greater. Centripetal force on the particle = mv2/R = m42R2/T2R = m42R/T2 = Bev So Bev = mv2/R and therefore v = BeR/m Frequency = 1/T = v/2R = [BeR/m]/ 2R = Be/2m and this is independent ...
... It is interesting to consider the frequency of the accelerating voltage as the energy of the particles gets greater. Centripetal force on the particle = mv2/R = m42R2/T2R = m42R/T2 = Bev So Bev = mv2/R and therefore v = BeR/m Frequency = 1/T = v/2R = [BeR/m]/ 2R = Be/2m and this is independent ...
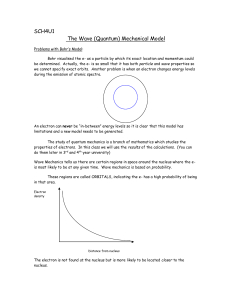
3.4oquantum.4u
... An electron can never be “in-between” energy levels so it is clear that this model has limitations and a new model needs to be generated. The study of quantum mechanics is a branch of mathematics which studies the properties of electrons. In this class we will use the results of the calculations. (Y ...
... An electron can never be “in-between” energy levels so it is clear that this model has limitations and a new model needs to be generated. The study of quantum mechanics is a branch of mathematics which studies the properties of electrons. In this class we will use the results of the calculations. (Y ...
PPTX
... interact differently with it: • Pions and protons can undergo nuclear interactions • This is because they have quarks inside, which can interact with quarks and gluons in the atoms of the media ...
... interact differently with it: • Pions and protons can undergo nuclear interactions • This is because they have quarks inside, which can interact with quarks and gluons in the atoms of the media ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.