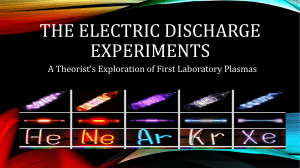
Electrons in Atoms - Brunswick City Schools / Homepage
... • Certain elements emit visible light when heated into a flame. • This chemical behavior is related to the arrangement of the electrons in its atom. ...
... • Certain elements emit visible light when heated into a flame. • This chemical behavior is related to the arrangement of the electrons in its atom. ...
Ch 11 WS Orbitals and Electron Arrangement
... 9. Principal energy levels are assigned values in order of ______________________ energy: n = 1, 2, 3, 4, and so forth. 10. In the quantum mechanical model the regions where electrons are likely to be found are called ______________________ and are denoted by______________________ . ...
... 9. Principal energy levels are assigned values in order of ______________________ energy: n = 1, 2, 3, 4, and so forth. 10. In the quantum mechanical model the regions where electrons are likely to be found are called ______________________ and are denoted by______________________ . ...
Lecture.1.part1
... certain particles (with this charge) interact. If the particles don’t interact in the prescribed way, they don’t have charge. The force, F, between two charges (and the classical mathematical model, Coulomb’s Law, kQ1Q2/r2), was derived experimentally. Subsequent to this we developed the ideas of el ...
... certain particles (with this charge) interact. If the particles don’t interact in the prescribed way, they don’t have charge. The force, F, between two charges (and the classical mathematical model, Coulomb’s Law, kQ1Q2/r2), was derived experimentally. Subsequent to this we developed the ideas of el ...
Matthew Jones - Phys 378 Web page:
... Kinetic theory of gases (Bernoulli, 1738) Boscovich: gasses composed of massive, point like particles with central forces No quantum mechanics but otherwise similar to the way we think of particle physics Forces turn out to be a consequence of the exchange of virtual quanta ...
... Kinetic theory of gases (Bernoulli, 1738) Boscovich: gasses composed of massive, point like particles with central forces No quantum mechanics but otherwise similar to the way we think of particle physics Forces turn out to be a consequence of the exchange of virtual quanta ...
Nuclear Chemistry - sullivanchem-ap
... 1. D—The mass should be 226 – (4 + 4 + 0 + 4) = 214. The atomic number should be 88 – (2 + 2 – 1 + 2) ...
... 1. D—The mass should be 226 – (4 + 4 + 0 + 4) = 214. The atomic number should be 88 – (2 + 2 – 1 + 2) ...
GeomagneticallyTrappedRadiation
... angles will be precipitated Drift loss cone Charge dependent drift |B| lowest over South Atlantic Anomaly (SAA) Particle can drift into region of low |B| and precipitate ...
... angles will be precipitated Drift loss cone Charge dependent drift |B| lowest over South Atlantic Anomaly (SAA) Particle can drift into region of low |B| and precipitate ...
example8
... (b) The photoelectric effect is the emission of electrons from a metal surface when electromagnetic radiation is shone onto the metal. For this to happen the frequency of the electromagnetic radiation must be above a certain minimum frequency known as the threshold frequency. If the frequency is bel ...
... (b) The photoelectric effect is the emission of electrons from a metal surface when electromagnetic radiation is shone onto the metal. For this to happen the frequency of the electromagnetic radiation must be above a certain minimum frequency known as the threshold frequency. If the frequency is bel ...
3-CProvencher
... • “The study of the electrical properties of gases seems to offer the most promising field for investigating the Nature of Electricity and Matter, for thanks to the Kinetic Theory of Gases our idea of non-electric processes in gases is much more vivid than they are for liquids or solids”– ...
... • “The study of the electrical properties of gases seems to offer the most promising field for investigating the Nature of Electricity and Matter, for thanks to the Kinetic Theory of Gases our idea of non-electric processes in gases is much more vivid than they are for liquids or solids”– ...
1/3
... Recall, Rutherford determined that the atom must contain a dense core of positive charge to account for the large angular deflections of incoming alpha particles. Also, as we discussed earlier, in order to probe matter of size, say A, the wavelength which you use to probe it must be at least this si ...
... Recall, Rutherford determined that the atom must contain a dense core of positive charge to account for the large angular deflections of incoming alpha particles. Also, as we discussed earlier, in order to probe matter of size, say A, the wavelength which you use to probe it must be at least this si ...
Acceleration at Shocks Without Particle Scattering
... – This would vary along the shock since it depends on the local shock normal angle (as known from past studies) ...
... – This would vary along the shock since it depends on the local shock normal angle (as known from past studies) ...
**DO NOT WRITE ON THIS PAPER
... 11. Match the following particles with their location in the atom. PARTICLE DESCRIPTION A. Electron I. In the nucleus B. Neutron II. In the nucleus C. Proton III. Move around outside the nucleus 12. Atoms are EXTREMELY small. How many atoms can you fit into the previous sentence? 13. If you put a g ...
... 11. Match the following particles with their location in the atom. PARTICLE DESCRIPTION A. Electron I. In the nucleus B. Neutron II. In the nucleus C. Proton III. Move around outside the nucleus 12. Atoms are EXTREMELY small. How many atoms can you fit into the previous sentence? 13. If you put a g ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 10. Write down the Dirac matrices in terms of the (2x2) Pauli spin matrices and unit matrix PART B ...
... 10. Write down the Dirac matrices in terms of the (2x2) Pauli spin matrices and unit matrix PART B ...
History of the Atom
... History of the Atom • 440 BC – Democritus developed the original atomic concept – There exist indivisible particles called atoms (“a” meaning not; “tomos” meaning cut • There exist empty space between atoms • Atoms are completely solid • Atoms are homogeneous with no internal structure • Atoms diffe ...
... History of the Atom • 440 BC – Democritus developed the original atomic concept – There exist indivisible particles called atoms (“a” meaning not; “tomos” meaning cut • There exist empty space between atoms • Atoms are completely solid • Atoms are homogeneous with no internal structure • Atoms diffe ...
FEL and Accelerator Physics
... W E N lg Let's notice, that in a non-uniform undultor, the fraction of the energy transformed to radiation, can be considerably increased using the capture of particles in the wave field in an autophasing mode. ...
... W E N lg Let's notice, that in a non-uniform undultor, the fraction of the energy transformed to radiation, can be considerably increased using the capture of particles in the wave field in an autophasing mode. ...
Accelerators - UC Davis Physics
... Electrically charged objects exert forces on each other -- opposite charges attract; like charges repel. •Coulomb’s law F = -K q1 q2 / r2 •Newton’s Law ...
... Electrically charged objects exert forces on each other -- opposite charges attract; like charges repel. •Coulomb’s law F = -K q1 q2 / r2 •Newton’s Law ...
Work sheet –chapter 2 CLASS - XI CHEMISTRY (Structure of Atom
... 7. Calculate energy of 2mole of photons of radiation whose frequency is 51014Hz. 8. What is emission and absorption spectra? 9. What transition in the hydrogen spectrum would have the same wavelength as the Balmer transition, n = 4 to n = 2 of He+ spectrum? 10. Spectral lines are regarded as the fi ...
... 7. Calculate energy of 2mole of photons of radiation whose frequency is 51014Hz. 8. What is emission and absorption spectra? 9. What transition in the hydrogen spectrum would have the same wavelength as the Balmer transition, n = 4 to n = 2 of He+ spectrum? 10. Spectral lines are regarded as the fi ...
Artificial Radioactivity Artificial Radioactivity And Q
... materials, they lose energy by two mechanisms: Collision loss, where energy is given to electrons in the atoms of the material, and radiation loss involving the conversion of electron kinetic energy to photons of Xradiation in the field of an atomic nucleus. As the incident electron traveling throug ...
... materials, they lose energy by two mechanisms: Collision loss, where energy is given to electrons in the atoms of the material, and radiation loss involving the conversion of electron kinetic energy to photons of Xradiation in the field of an atomic nucleus. As the incident electron traveling throug ...
Problem set 5
... 3. Obtain a matrix representation of the Clebsch-Gordan coefficients for the transformation from uncoupled to coupled basis of a system of two spin-half particles. Show that the matrix is unitary. Specify the uncoupled and coupled basis vectors and their orderings. 4. Suppose J~ = ~L + S~ is the sum ...
... 3. Obtain a matrix representation of the Clebsch-Gordan coefficients for the transformation from uncoupled to coupled basis of a system of two spin-half particles. Show that the matrix is unitary. Specify the uncoupled and coupled basis vectors and their orderings. 4. Suppose J~ = ~L + S~ is the sum ...
Chapter 7 The Quantum- Mechanical Model of the
... uncertainties in both the position and speed of a particle was inversely proportional to its mass. – x = position, ∆x = uncertainty in position – v = velocity, ∆v = uncertainty in velocity – m = mass ...
... uncertainties in both the position and speed of a particle was inversely proportional to its mass. – x = position, ∆x = uncertainty in position – v = velocity, ∆v = uncertainty in velocity – m = mass ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.