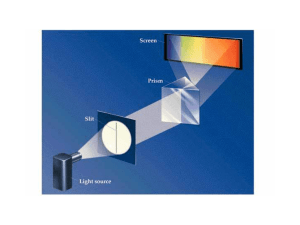
making a bohr model - hrsbstaff.ednet.ns.ca
... In 1913, Neils Bohr proposed his model of the atom. This pictured the atom as having a dense, positively charged nucleus and negatively charged electrons in specific orbits, also called energy levels or shells, around the nucleus. Because of its simplicity and general ability to explain chemical cha ...
... In 1913, Neils Bohr proposed his model of the atom. This pictured the atom as having a dense, positively charged nucleus and negatively charged electrons in specific orbits, also called energy levels or shells, around the nucleus. Because of its simplicity and general ability to explain chemical cha ...
chapter 7: atomic structure and periodicity
... a) Calculate the de Broglie wavelength of a neutron (1.675 x 10-27 kg) moving at 1.00% of the speed of light. b) Calculate the velocity of a neutron with a wavelength of 75 pm. 2. Calculate the wavelength of light emitted when the following transition occurs in the hydrogen atom: a) n = 4 n = 2 . ...
... a) Calculate the de Broglie wavelength of a neutron (1.675 x 10-27 kg) moving at 1.00% of the speed of light. b) Calculate the velocity of a neutron with a wavelength of 75 pm. 2. Calculate the wavelength of light emitted when the following transition occurs in the hydrogen atom: a) n = 4 n = 2 . ...
do with electron orbitals?
... III. The location of the electron is _______ a. same, same, same b. same, same, different c. same, different, different d. different, same, different e. different, different, different ...
... III. The location of the electron is _______ a. same, same, same b. same, same, different c. same, different, different d. different, same, different e. different, different, different ...
Chemistry 1 Practice Final Exam - Tutor
... b) A hydrogen atom in the ground state (n = 1) absorbs a 102.6 nm photon. What is the principal quantum number, n, of the excited state after this transition? The energy levels of the H atoms are given by: ...
... b) A hydrogen atom in the ground state (n = 1) absorbs a 102.6 nm photon. What is the principal quantum number, n, of the excited state after this transition? The energy levels of the H atoms are given by: ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 1. Prove [ [A,B], C]+[ [B,C], A]+[ [C, A], B] = 0 2. State Heisenberg’s uncertainty principle 3. What are spherical harmonics? Are they mutually orthogonal? 4. Prove that the square of the angular momentum commutes with its z-component. 5. If A and B are two operators, then show that [A-1[A,B]] = 2B ...
... 1. Prove [ [A,B], C]+[ [B,C], A]+[ [C, A], B] = 0 2. State Heisenberg’s uncertainty principle 3. What are spherical harmonics? Are they mutually orthogonal? 4. Prove that the square of the angular momentum commutes with its z-component. 5. If A and B are two operators, then show that [A-1[A,B]] = 2B ...
Some properties of Thomson`s atom
... In Thomson’s model for the Hydrogen atom, the positive charge e is uniformly distributed within a sfere of radius a0 . The electron, having charge −e, is considered to be a point particle and is located inside the sphere. a) Find the electric field and the potential generated by the positive charge ...
... In Thomson’s model for the Hydrogen atom, the positive charge e is uniformly distributed within a sfere of radius a0 . The electron, having charge −e, is considered to be a point particle and is located inside the sphere. a) Find the electric field and the potential generated by the positive charge ...
Modern physics 2330
... 5- (..) A black hole is an object of sufficiently high density can trap light forever. 6- ( ) Millikan’s oil drop experiment is inaccurate because it is difficult to measure the speed of drop. 7- (..) Thompson cloud chamber experiment is inaccurate because the theory applies only to a single particl ...
... 5- (..) A black hole is an object of sufficiently high density can trap light forever. 6- ( ) Millikan’s oil drop experiment is inaccurate because it is difficult to measure the speed of drop. 7- (..) Thompson cloud chamber experiment is inaccurate because the theory applies only to a single particl ...
Chemistry 11 Early Models of the Atom Power Point
... the electron and measured its e/m (charge to mass) ratio. ...
... the electron and measured its e/m (charge to mass) ratio. ...
Atomic models
... 3) Calculations by Rutherford showed that the radius of the atom is about 10 –10 m, while that of nucleus is 10–15 m. Rutherford’s Nuclear Model of Atom (was proposed after the discovery of protons)– based on above observations and conclusions 1) The positive charge and most of the mass of the atom ...
... 3) Calculations by Rutherford showed that the radius of the atom is about 10 –10 m, while that of nucleus is 10–15 m. Rutherford’s Nuclear Model of Atom (was proposed after the discovery of protons)– based on above observations and conclusions 1) The positive charge and most of the mass of the atom ...
AP Chemistry Chapter 6 Outline for Concepts to Know 6.1 Wave
... electrons moving from excited to ground (or less excited) levels Calculations of energy states of hydrogen and other atoms will NOT be tested Bohr model will not be specifically tested 6.4Wave Behavior of Matter Conceptual understanding that all matter has an energy- and a wave-equivalent, but ...
... electrons moving from excited to ground (or less excited) levels Calculations of energy states of hydrogen and other atoms will NOT be tested Bohr model will not be specifically tested 6.4Wave Behavior of Matter Conceptual understanding that all matter has an energy- and a wave-equivalent, but ...
Quantum Numbers (and their meaning)
... m states is always odd (2 + 1) and should produce odd number of ...
... m states is always odd (2 + 1) and should produce odd number of ...
The Meaning of the Wave Function
... To explain the properties of atoms we need to understand how electrons are arranged in the orbitals the wave function gives us. It is the arrangements of the electrons that determine the chemical properties of an atom. There are three rules that govern the way electrons fill orbitals: The Pauli excl ...
... To explain the properties of atoms we need to understand how electrons are arranged in the orbitals the wave function gives us. It is the arrangements of the electrons that determine the chemical properties of an atom. There are three rules that govern the way electrons fill orbitals: The Pauli excl ...
File
... According to the quantum theory of an atom, in an orbital a. an electron's position cannot be known precisely. b. an electron has no energy. c. electrons cannot be found. d. electrons travel around the nucleus on paths of specific radii. ...
... According to the quantum theory of an atom, in an orbital a. an electron's position cannot be known precisely. b. an electron has no energy. c. electrons cannot be found. d. electrons travel around the nucleus on paths of specific radii. ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).