Frank-Hertz experiment with Neon
... electric discharges through various low-pressure gasses in evacuated glass tubes had shown that atoms will only emit light (that is, electromagnetic radiation) at certain discrete frequencies. To overcome this difficulty, Niels Bohr proposed in 1913 the now called Bohr model of the atom, which sugge ...
... electric discharges through various low-pressure gasses in evacuated glass tubes had shown that atoms will only emit light (that is, electromagnetic radiation) at certain discrete frequencies. To overcome this difficulty, Niels Bohr proposed in 1913 the now called Bohr model of the atom, which sugge ...
The Interaction of Radiation and Matter: Quantum
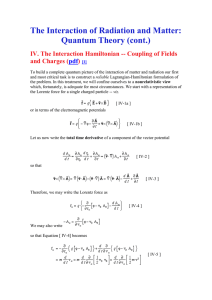
... Electric fields of interest vary only slightly over an atom so that we should be able to expand the external field in a rapidly converging series as follows [ IV-16 ] Substituting this expansion into Equation [ IV-15b ] and integrating we obtain [ IV-17a ] Formally, we may cast this expansion for th ...
... Electric fields of interest vary only slightly over an atom so that we should be able to expand the external field in a rapidly converging series as follows [ IV-16 ] Substituting this expansion into Equation [ IV-15b ] and integrating we obtain [ IV-17a ] Formally, we may cast this expansion for th ...
Physics 834: Problem Set #4
... 5. (10 pts) The Stark effect describes the energy shift of atomic energy levels due to applied electric fields. The differential equation describing this effect may be written ...
... 5. (10 pts) The Stark effect describes the energy shift of atomic energy levels due to applied electric fields. The differential equation describing this effect may be written ...
Atomic Theory Practice Test
... Identify the letter of the choice that best completes the statement or answers the question. ____ 18. The electrons involved in the formation of a chemical bond are called a. dipoles. c. Lewis electrons. b. s electrons. d. valence electrons. ____ 19. In a chemical bond, the link between atoms result ...
... Identify the letter of the choice that best completes the statement or answers the question. ____ 18. The electrons involved in the formation of a chemical bond are called a. dipoles. c. Lewis electrons. b. s electrons. d. valence electrons. ____ 19. In a chemical bond, the link between atoms result ...
Atomic Structure (history of atom)
... Foil surrounded by fluorescent screen which flashed when hit by an a-particle Experiment done in a vacuum ...
... Foil surrounded by fluorescent screen which flashed when hit by an a-particle Experiment done in a vacuum ...
manual
... ψ indicates wave function, and it is the amplitude of the wave related to the motion of a particle. In particular, the wave function in a one-electron picture is called orbital. It can have positive, negative or zero values. The points or regions with the zero amplitude of the wave function are call ...
... ψ indicates wave function, and it is the amplitude of the wave related to the motion of a particle. In particular, the wave function in a one-electron picture is called orbital. It can have positive, negative or zero values. The points or regions with the zero amplitude of the wave function are call ...
atomicspectra1-2
... give a total orbital angular momentum L = S ili. • (b) The spins of the electrons are coupled to give a total spin S = Si si. • combination of a particular S value with a particular L value , a spectroscopic term 2S+1L.(2S + 1 is the multiplicity of the term) • total angular momentum, J = S + L : le ...
... give a total orbital angular momentum L = S ili. • (b) The spins of the electrons are coupled to give a total spin S = Si si. • combination of a particular S value with a particular L value , a spectroscopic term 2S+1L.(2S + 1 is the multiplicity of the term) • total angular momentum, J = S + L : le ...
Another version - Scott Aaronson
... formal model of CTC computation, and showed that in both the classical and quantum cases, it has exactly the power of PSPACE (believed to be even larger than NP) ...
... formal model of CTC computation, and showed that in both the classical and quantum cases, it has exactly the power of PSPACE (believed to be even larger than NP) ...
Quantum computing and the monogamy of entanglement
... • M.A. Nielsen and I.L. Chuang, Quantum Computation and Quantum Information, Cambridge University Press, 2000. ...
... • M.A. Nielsen and I.L. Chuang, Quantum Computation and Quantum Information, Cambridge University Press, 2000. ...
instroduction_a_final
... Concepts: ----- We are going to use these terms all the time. Just remember them. 1. Wavefunctions: ----It is mathematical function that contains a complete description of the system. If we know the wavefunction we can calculate the properties of the system. For example: a mathematical function of o ...
... Concepts: ----- We are going to use these terms all the time. Just remember them. 1. Wavefunctions: ----It is mathematical function that contains a complete description of the system. If we know the wavefunction we can calculate the properties of the system. For example: a mathematical function of o ...
CHEMISTRY MIDTERM REVIEW
... 22. Nitrogen-13 emits beta radiation and decays to Carbon-13 with a half-life of 10 min. Assuming a starting mass of 2.00 grams of Nitrogen-13, how many grams will be present at the end of three half-lives? 23. Write an equation for the alpha decay of Pa-231. 24. Write an equation for the beta decay ...
... 22. Nitrogen-13 emits beta radiation and decays to Carbon-13 with a half-life of 10 min. Assuming a starting mass of 2.00 grams of Nitrogen-13, how many grams will be present at the end of three half-lives? 23. Write an equation for the alpha decay of Pa-231. 24. Write an equation for the beta decay ...
Phys202_Final_Exam_Spr2007.doc
... 32. The equation for the magnetic field in terms of an infinitesimal current segment a distance r away is whose equation: a. Faraday’s b. ~ Biot & Savart’s c Amperes d. Gauss’ ...
... 32. The equation for the magnetic field in terms of an infinitesimal current segment a distance r away is whose equation: a. Faraday’s b. ~ Biot & Savart’s c Amperes d. Gauss’ ...
here - Dalibor Hrg
... 2. Calculate the function in these arguments so we have all results in n qubits. 2 n 1 ...
... 2. Calculate the function in these arguments so we have all results in n qubits. 2 n 1 ...
20040929114512301
... – Decoherence is an interesting problem: heating rates of seconds gives loads of time for gates. – Quantum memories are harder to realize: few qubit applications? ...
... – Decoherence is an interesting problem: heating rates of seconds gives loads of time for gates. – Quantum memories are harder to realize: few qubit applications? ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 1. Find the determinant value of the Loerntz transformation matrix. 2. State the relation between relativistic energy and relativistic momentum. 3. Define 4-current and write down the continuity equation in terms of it. 4. State the covariant form of Lorentz force equation. 5. Define differential sc ...
... 1. Find the determinant value of the Loerntz transformation matrix. 2. State the relation between relativistic energy and relativistic momentum. 3. Define 4-current and write down the continuity equation in terms of it. 4. State the covariant form of Lorentz force equation. 5. Define differential sc ...
Study Material 1
... Mass Number (A) :Sum of the number of protons and neutrons present in thenucleus. Thomson model of an atom: This model proposed that atom is considered asa uniform positively charged sphere and electrons are embedded in it.An important feature of Thomson model of an atom was that mass of atom iscons ...
... Mass Number (A) :Sum of the number of protons and neutrons present in thenucleus. Thomson model of an atom: This model proposed that atom is considered asa uniform positively charged sphere and electrons are embedded in it.An important feature of Thomson model of an atom was that mass of atom iscons ...
Balancing Chemical Equations
... both sides must be the same for the activity to work properly. Therefore you must change the amount of atoms in each chemical to balance the sides. ...
... both sides must be the same for the activity to work properly. Therefore you must change the amount of atoms in each chemical to balance the sides. ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).