Atomic Physics 4
... – This is a list of different videos explaining (some with examples) of the Compton scattering model. ...
... – This is a list of different videos explaining (some with examples) of the Compton scattering model. ...
Practice Test 1 (Chapters 1-7)
... 31.The total number of atoms represented by the a. vodka formula Co3(PO4)2 is b. oily water a. 13 c. soil (dust) b. 11 d. sodium chloride c. 8 e. aluminum d. 17 26. Which of the following processes is a chemical e. 6 change? 32. Which atomic particle determines the chemical a. Dry ice sublimes when ...
... 31.The total number of atoms represented by the a. vodka formula Co3(PO4)2 is b. oily water a. 13 c. soil (dust) b. 11 d. sodium chloride c. 8 e. aluminum d. 17 26. Which of the following processes is a chemical e. 6 change? 32. Which atomic particle determines the chemical a. Dry ice sublimes when ...
CHAPTER 2 STRUCTURE OF ATOM • Atom is the smallest
... missing and come as dark lines. The study of emission or absorption spectra is referred as spectroscopy. Spectral Lines for atomic hydrogen: ...
... missing and come as dark lines. The study of emission or absorption spectra is referred as spectroscopy. Spectral Lines for atomic hydrogen: ...
Energy Levels for the Hydrogen Atom (from Ph234)
... perturbation to the system we are considering. For an isolated atom in an excited energy state, there is no such external perturbation. From the formalism we have used up until now, we would say that if the atom is in an eigenstate, then it is in a “stationary state”, and the wavefunction will evolv ...
... perturbation to the system we are considering. For an isolated atom in an excited energy state, there is no such external perturbation. From the formalism we have used up until now, we would say that if the atom is in an eigenstate, then it is in a “stationary state”, and the wavefunction will evolv ...
Solutions - Dynamic Science
... Electrons and protons only. Protons and electrons only. Neutrons, protons and electrons. ...
... Electrons and protons only. Protons and electrons only. Neutrons, protons and electrons. ...
Exam 2 (word)
... a) there aren’t enough charged particles in the dielectric to cancel the free charges on the plate b) the charges run into obstacles as they move through the dielectric c) the resonance of the charged dipoles isn’t sustained for a long enough time d) the polarization of the dielectric isn’t enough t ...
... a) there aren’t enough charged particles in the dielectric to cancel the free charges on the plate b) the charges run into obstacles as they move through the dielectric c) the resonance of the charged dipoles isn’t sustained for a long enough time d) the polarization of the dielectric isn’t enough t ...
Chap. 7 - Quantum Chemistry
... frequency, the distance between the peaks of the wave must be small (short wavelength). In summary, an inverse relationship exists between frequency and wavelength of electromagnetic radiation as the speed of light is always constant. ...
... frequency, the distance between the peaks of the wave must be small (short wavelength). In summary, an inverse relationship exists between frequency and wavelength of electromagnetic radiation as the speed of light is always constant. ...
Document
... ” …not a mechanical influence … … an influence on the very conditions which define the possible types of predictions regarding the future behavior of the system.” ...
... ” …not a mechanical influence … … an influence on the very conditions which define the possible types of predictions regarding the future behavior of the system.” ...
P202 Lecture 2
... Major differences between the “QM” hydrogen atom and Bohr’s model (my list): •The electrons do not travel in orbits, but in well defined states (orbitals) that have particular shapes (probability distributions for the electrons, or linear combinations thereof) [9 responses] •The Energy levels are NO ...
... Major differences between the “QM” hydrogen atom and Bohr’s model (my list): •The electrons do not travel in orbits, but in well defined states (orbitals) that have particular shapes (probability distributions for the electrons, or linear combinations thereof) [9 responses] •The Energy levels are NO ...
Fall Exam 1
... showed that an electron could have C. demonstrated the existence of more than one charge. neutrons. B. proved that Thomson’s “plum D. determined the charge on a single pudding” model of the atom’s electron. structure was correct. 19. Nobel prize winner Ernest Rutherford conducted an experiment with ...
... showed that an electron could have C. demonstrated the existence of more than one charge. neutrons. B. proved that Thomson’s “plum D. determined the charge on a single pudding” model of the atom’s electron. structure was correct. 19. Nobel prize winner Ernest Rutherford conducted an experiment with ...
(p. 522)
... 7. Hydrogen forms metallic (interstitial) hydrides with the d and f transition elements. Which of the following statements is correct? (p. 567) C A.These substances have distinct stoichiometric formulas like ionic hydrides. B.Hydrogen forms bonds with the metals by donating its electron to the valen ...
... 7. Hydrogen forms metallic (interstitial) hydrides with the d and f transition elements. Which of the following statements is correct? (p. 567) C A.These substances have distinct stoichiometric formulas like ionic hydrides. B.Hydrogen forms bonds with the metals by donating its electron to the valen ...
Postulate 1
... quantum mechanical problems does enable us to calculate from the Schrödinger equation the correct values for eigenvalues which specify energy and momentum values. However, the use of normalized wave functions does afford both mathematical and conceptual advantages. In particular, “probabilities” are ...
... quantum mechanical problems does enable us to calculate from the Schrödinger equation the correct values for eigenvalues which specify energy and momentum values. However, the use of normalized wave functions does afford both mathematical and conceptual advantages. In particular, “probabilities” are ...
Electron Configurations
... • Wave nature is inversely related to mass so we don’t notice wave nature of large objects. • However, electrons have a small mass and the wave characteristic is more noticable ...
... • Wave nature is inversely related to mass so we don’t notice wave nature of large objects. • However, electrons have a small mass and the wave characteristic is more noticable ...
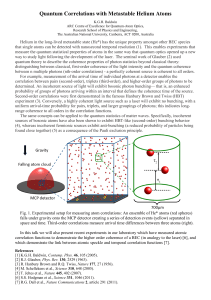
Quantum Correlations with Metastable Helium Atoms
... Second-order correlations were first demonstrated in the famous Hanbury Brown and Twiss (HBT) experiment (3). Conversely, a highly coherent light source such as a laser will exhibit no bunching, with a uniform arrival-time probability for pairs, triplets, and larger groupings of photons; this indica ...
... Second-order correlations were first demonstrated in the famous Hanbury Brown and Twiss (HBT) experiment (3). Conversely, a highly coherent light source such as a laser will exhibit no bunching, with a uniform arrival-time probability for pairs, triplets, and larger groupings of photons; this indica ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).