IS BOHR`S CHALLENGE STILL RELEVANT?
... raises issue of non-locality. However, this non-locality is confined to the atom and the moment of the leap and is not related to the connection between different events. Consequently, it is not the same non-locality as that of entangled states, for example. Nevertheless the existence of this “hidde ...
... raises issue of non-locality. However, this non-locality is confined to the atom and the moment of the leap and is not related to the connection between different events. Consequently, it is not the same non-locality as that of entangled states, for example. Nevertheless the existence of this “hidde ...
Quantum Correlations with Metastable Helium Atoms
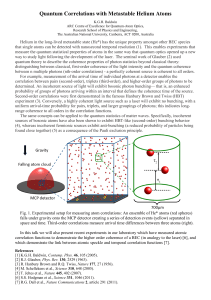
... Second-order correlations were first demonstrated in the famous Hanbury Brown and Twiss (HBT) experiment (3). Conversely, a highly coherent light source such as a laser will exhibit no bunching, with a uniform arrival-time probability for pairs, triplets, and larger groupings of photons; this indica ...
... Second-order correlations were first demonstrated in the famous Hanbury Brown and Twiss (HBT) experiment (3). Conversely, a highly coherent light source such as a laser will exhibit no bunching, with a uniform arrival-time probability for pairs, triplets, and larger groupings of photons; this indica ...
Grade 11 Chemistry E.. - hrsbstaff.ednet.ns.ca
... (a) How many moles of hydrogen are needed to completely react with two moles of nitrogen? (b) How many grams of hydrogen are necessary to react completely with 50.0 g of nitrogen? 33. Write and balance the chemical equation: Sodium hydroxide and hydrochloric acid combine to make table salt and water ...
... (a) How many moles of hydrogen are needed to completely react with two moles of nitrogen? (b) How many grams of hydrogen are necessary to react completely with 50.0 g of nitrogen? 33. Write and balance the chemical equation: Sodium hydroxide and hydrochloric acid combine to make table salt and water ...
White Dwarf Properties and the Degenerate Electron Gas
... The first two white dwarfs to be discovered were 40 Eridani B and Sirius B. Observations revealed these stars to be of a type fundamentally different to the ‘ordinary’ stars, and over several decades at the start of the twentieth century the theory of stellar structure was revised to incorporate thi ...
... The first two white dwarfs to be discovered were 40 Eridani B and Sirius B. Observations revealed these stars to be of a type fundamentally different to the ‘ordinary’ stars, and over several decades at the start of the twentieth century the theory of stellar structure was revised to incorporate thi ...
Lecture 3 Teaching notes
... In a many-particle system, the key question is: how do the electrons occupy the states we have found? Note that the term “state” is unfortunately used in two senses: The possible wavefunctions for one particle, and the total wavefunction for all the electrons of a many-electron system. I will try to ...
... In a many-particle system, the key question is: how do the electrons occupy the states we have found? Note that the term “state” is unfortunately used in two senses: The possible wavefunctions for one particle, and the total wavefunction for all the electrons of a many-electron system. I will try to ...
Review
... 1) the potential energy doesn’t change with time, V(x,t) = V(x) 2) the wavefunction is separable and can be expressed as the product of a spatial function and a temporal function, ( x , t ) ( x )(t ) By making these substitutions into the time-dependent equation, the time-independent equation ...
... 1) the potential energy doesn’t change with time, V(x,t) = V(x) 2) the wavefunction is separable and can be expressed as the product of a spatial function and a temporal function, ( x , t ) ( x )(t ) By making these substitutions into the time-dependent equation, the time-independent equation ...
atomic clock - National Physical Laboratory
... Time measurement has become a basic part of everyday life and accuracies of the nearest minute or a few seconds are usually good enough for most human activities, but highly accurate timing plays a vital role in many other aspects of the modern world. ...
... Time measurement has become a basic part of everyday life and accuracies of the nearest minute or a few seconds are usually good enough for most human activities, but highly accurate timing plays a vital role in many other aspects of the modern world. ...
Orbitals
... 1. No electrons are emitted if the frequency of light used is less than νo, regardless of the intensity of the light. 2. For light with a frequency≥ νo , electrons are emitted. The number of electrons increases with the intensity of the light. 3. For light with a frequency > νo , the electrons are e ...
... 1. No electrons are emitted if the frequency of light used is less than νo, regardless of the intensity of the light. 2. For light with a frequency≥ νo , electrons are emitted. The number of electrons increases with the intensity of the light. 3. For light with a frequency > νo , the electrons are e ...
Physics 882: Problem Set 2 Due Friday, January 24, 2002
... (| ↑↓> −| ↓↑>) / 2. Show that this state is an eigenstate of the operator S 2 with eigenvalue S(S + 1) = 0, and of the operator Sz with eigenvalue 0, where S 2 is the operator representing the square of the total spin, and Sz is the z component of total spin. 3. This problem is based on the notes ha ...
... (| ↑↓> −| ↓↑>) / 2. Show that this state is an eigenstate of the operator S 2 with eigenvalue S(S + 1) = 0, and of the operator Sz with eigenvalue 0, where S 2 is the operator representing the square of the total spin, and Sz is the z component of total spin. 3. This problem is based on the notes ha ...
Liquid-drop model of electron and atom
... of atom. The idea of the Bohr orbits of electron in the atom thus was born. This idea assumes that the electrons revolve around the positively charged nucleus, being found in specific orbits. Passage from one orbit to another is accompanied by the emission of the quanta of the electromagnetic radiat ...
... of atom. The idea of the Bohr orbits of electron in the atom thus was born. This idea assumes that the electrons revolve around the positively charged nucleus, being found in specific orbits. Passage from one orbit to another is accompanied by the emission of the quanta of the electromagnetic radiat ...
Lecture 4 - Indiana University Bloomington
... SCF (Self Consistent Field) Method (a.ka. Mean Field or Hartree Fock) ...
... SCF (Self Consistent Field) Method (a.ka. Mean Field or Hartree Fock) ...
Two-electron Interference
... Very much like the ubiquitous quantum interference of a single particle with itself, quantum interference of two independent, but indistinquishable, particles is also possible. For a single particle, the interference is between the amplitudes of the particle’s wave function, whereas the interfere ...
... Very much like the ubiquitous quantum interference of a single particle with itself, quantum interference of two independent, but indistinquishable, particles is also possible. For a single particle, the interference is between the amplitudes of the particle’s wave function, whereas the interfere ...
Isotopic indistinguishibility, scattering processes and the non mass
... rue Cuvier, 75005 Paris ([email protected]). Introduction: Numerous theoretical models have been proposed to explain the non mass dependent fractionation for oxygen isotopes observed during the synthesis of ozone [1]. The most accepted theory is based on a modification of the standard RRKM model of uni ...
... rue Cuvier, 75005 Paris ([email protected]). Introduction: Numerous theoretical models have been proposed to explain the non mass dependent fractionation for oxygen isotopes observed during the synthesis of ozone [1]. The most accepted theory is based on a modification of the standard RRKM model of uni ...
Lecture 12 Atomic structure
... Since single-particle Hamiltonian Ĥ0 continues to commute with the angular momentum operator, [Ĥ0 , L̂] = 0, its eigenfunctions remain indexed by quantum numbers (n, #, m! , ms ). However, since effective potential, V (r ) + Ui (r ), is no longer Coulomb-like, # values for a given n need not be de ...
... Since single-particle Hamiltonian Ĥ0 continues to commute with the angular momentum operator, [Ĥ0 , L̂] = 0, its eigenfunctions remain indexed by quantum numbers (n, #, m! , ms ). However, since effective potential, V (r ) + Ui (r ), is no longer Coulomb-like, # values for a given n need not be de ...
PHY492: Nuclear & Particle Physics Lecture 4 Nature of the nuclear force
... Nuclear mass and binding energy The mass of a bound system is always less than the mass of its component parts. For example, the mass of the hydrogen atom is 13.5 eV/c2 less than proton mass plus electron mass. When the hydrogen atom is formed, 13.5 eV is released in photons. mH c 2 − m p + me c 2 ...
... Nuclear mass and binding energy The mass of a bound system is always less than the mass of its component parts. For example, the mass of the hydrogen atom is 13.5 eV/c2 less than proton mass plus electron mass. When the hydrogen atom is formed, 13.5 eV is released in photons. mH c 2 − m p + me c 2 ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).