SPH 4U - mackenziekim
... How much kinetic energy does the electron have? 3.4 eV or 5.4x10-19 J How much energy does it take to ionize the hydrogen atom from this energy level (binding energy)? 3.4 eV or 5.4x10-19 J -ve ...
... How much kinetic energy does the electron have? 3.4 eV or 5.4x10-19 J How much energy does it take to ionize the hydrogen atom from this energy level (binding energy)? 3.4 eV or 5.4x10-19 J -ve ...
The Chemical Basis of Life I: Atoms, Molecules, and Water
... Isotopes are different forms of the same element that differ in their number of neutrons. differ in their number of protons. are all produced artificially. cannot form covalent bonds. cannot form ions. ...
... Isotopes are different forms of the same element that differ in their number of neutrons. differ in their number of protons. are all produced artificially. cannot form covalent bonds. cannot form ions. ...
No Slide Title
... nucleus. In Bohr’s model, an electron can travel around a nucleus without radiating energy. Furthermore, an electron in a given orbit has a certain definite amount of energy. The only way an electron can lose energy is by dropping from one energy level to a lower one. When this happens, the atom emi ...
... nucleus. In Bohr’s model, an electron can travel around a nucleus without radiating energy. Furthermore, an electron in a given orbit has a certain definite amount of energy. The only way an electron can lose energy is by dropping from one energy level to a lower one. When this happens, the atom emi ...
Chemistry 11 – Course Outcomes
... State the postulates of Dalton’s atomic theory List which statements of Dalton’s theory we now believe to be incorrect Give observable evidence to support the idea that there are positive and negative charges. Describe force between like charges and opposite charges Explain how J.J. Thomson changed ...
... State the postulates of Dalton’s atomic theory List which statements of Dalton’s theory we now believe to be incorrect Give observable evidence to support the idea that there are positive and negative charges. Describe force between like charges and opposite charges Explain how J.J. Thomson changed ...
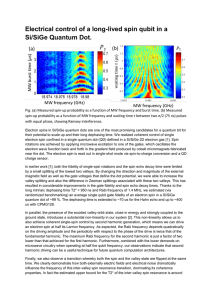
Electrical control of a long-lived spin qubit in a
... by a small splitting of the lowest two valleys. By changing the direction and magnitude of the external magnetic field as well as the gate voltages that define the dot potential, we were able to increase the valley splitting and also the difference in Zeeman splittings associated with these two vall ...
... by a small splitting of the lowest two valleys. By changing the direction and magnitude of the external magnetic field as well as the gate voltages that define the dot potential, we were able to increase the valley splitting and also the difference in Zeeman splittings associated with these two vall ...
7 - Physics at Oregon State University
... 2. Operator A describes a physical observable and acts on kets. 3. One of the eigenvalues an of A is the only possible result of a measurement. 4. The probability of obtaining the eigenvalue an : P an 5. State vector collapse : ' ...
... 2. Operator A describes a physical observable and acts on kets. 3. One of the eigenvalues an of A is the only possible result of a measurement. 4. The probability of obtaining the eigenvalue an : P an 5. State vector collapse : ' ...
Lorentz Invaiance Violation and Granularity of space time
... Let us take up the notion that space-time contains some granular/discrete aspect with characteristic scale given by MPlanck The lesson from the previous studies is that such structure, if exists, can not lead to breakdown of Lorentz Invariance. It is of course hard to envision something like that wh ...
... Let us take up the notion that space-time contains some granular/discrete aspect with characteristic scale given by MPlanck The lesson from the previous studies is that such structure, if exists, can not lead to breakdown of Lorentz Invariance. It is of course hard to envision something like that wh ...
Honors Chemistry Midterm Review 2008
... b. Mosely:- x-ray spectra; 1913; modern periodic table; arranged by atomic # c. Dobereiner: - 1817 Law of triads, Li Cl Na Br K I d. Berzelius:- Atomic weights 1828; chemical formula notation; first to use symbols and numbers e. Newlands: -law of octaves 1864; He arranged all the elements known at t ...
... b. Mosely:- x-ray spectra; 1913; modern periodic table; arranged by atomic # c. Dobereiner: - 1817 Law of triads, Li Cl Na Br K I d. Berzelius:- Atomic weights 1828; chemical formula notation; first to use symbols and numbers e. Newlands: -law of octaves 1864; He arranged all the elements known at t ...
G25.2666: Quantum Mechanics II
... in such a potential field. In practice, this is not a bad assumption since the mass of the proton is approximately 2000 time that of the electron. However, what happens when the “source” of the potential is not so heavy and can move on a time scale similar to that of the particle. An example would b ...
... in such a potential field. In practice, this is not a bad assumption since the mass of the proton is approximately 2000 time that of the electron. However, what happens when the “source” of the potential is not so heavy and can move on a time scale similar to that of the particle. An example would b ...
o Schrödinger equation for o Two-electron atoms. o Multi
... Orthohelium states are lower in energy than the parahelium states. Explanation for this is: 1. Parallel spins make the spin part of the wavefunction symmetric. 2. Total wavefunction for electrons must be antisymmetric since electrons are fermions. 3. This forces space part of wavefunction to be a ...
... Orthohelium states are lower in energy than the parahelium states. Explanation for this is: 1. Parallel spins make the spin part of the wavefunction symmetric. 2. Total wavefunction for electrons must be antisymmetric since electrons are fermions. 3. This forces space part of wavefunction to be a ...
Notes 2 Balancing
... those elements first. Delay the balancing of atoms (often hydrogen and oxygen) that appear in more that one reactant or product. • If a polyatomic ion appears on both sides of the equation, treat it as a single unit in you counts. ...
... those elements first. Delay the balancing of atoms (often hydrogen and oxygen) that appear in more that one reactant or product. • If a polyatomic ion appears on both sides of the equation, treat it as a single unit in you counts. ...
Document
... The Atom and Unanswered Questions • Recall that in Rutherford's model, the atom’s mass is concentrated in the nucleus and electrons move around it. • The model doesn’t explain how the electrons were arranged around the nucleus. • The model doesn’t explain why negatively charged electrons aren’t pul ...
... The Atom and Unanswered Questions • Recall that in Rutherford's model, the atom’s mass is concentrated in the nucleus and electrons move around it. • The model doesn’t explain how the electrons were arranged around the nucleus. • The model doesn’t explain why negatively charged electrons aren’t pul ...
CMC Chapter 05
... The Atom and Unanswered Questions • Recall that in Rutherford's model, the atom’s mass is concentrated in the nucleus and electrons move around it. • The model doesn’t explain how the electrons were arranged around the nucleus. • The model doesn’t explain why negatively charged electrons aren’t pul ...
... The Atom and Unanswered Questions • Recall that in Rutherford's model, the atom’s mass is concentrated in the nucleus and electrons move around it. • The model doesn’t explain how the electrons were arranged around the nucleus. • The model doesn’t explain why negatively charged electrons aren’t pul ...
CMC Chapter 05
... The Atom and Unanswered Questions • Recall that in Rutherford's model, the atom’s mass is concentrated in the nucleus and electrons move around it. • The model doesn’t explain how the electrons were arranged around the nucleus. • The model doesn’t explain why negatively charged electrons aren’t pul ...
... The Atom and Unanswered Questions • Recall that in Rutherford's model, the atom’s mass is concentrated in the nucleus and electrons move around it. • The model doesn’t explain how the electrons were arranged around the nucleus. • The model doesn’t explain why negatively charged electrons aren’t pul ...
Chapter 13 Spectroscopy NMR, IR, MS, UV-Vis
... C. Electromagnetic radiation of radio frequency wavelengths is of the right energy range to cause the nucleus to move (resonate) between these two energy states. This absorption allows detection of the hydrogen or carbon-13 nucleus. Different nuclei experiencing different magnetic fields and thus di ...
... C. Electromagnetic radiation of radio frequency wavelengths is of the right energy range to cause the nucleus to move (resonate) between these two energy states. This absorption allows detection of the hydrogen or carbon-13 nucleus. Different nuclei experiencing different magnetic fields and thus di ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).