* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download P202 Lecture 2
Renormalization group wikipedia , lookup
Schrödinger equation wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Spherical harmonics wikipedia , lookup
Tight binding wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Matter wave wikipedia , lookup
Wave–particle duality wikipedia , lookup
Electron configuration wikipedia , lookup
Atomic orbital wikipedia , lookup
Particle in a box wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
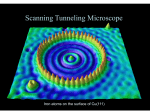
Schrodinger’s Equation Solutions Finite Square Well For this case, you must solve a transcendental equation to find the solutions that obey the boundary conditions (in particular, continuity of the function and its derivative at the well boundary). For the geometry we considered in class, this takes on one of two forms: Even solutions k=k tan(ka/2) ; Odd solutions k= -k cot(ka/2) Recall from class that we can recast the transcendental equation into a dimensionless form, where the controlling parameter is the ratio of the potential well depth to the “confinement energy”. You can get the spreadsheet for the even solutions from the website: http://physics.indiana.edu/~courses/p 301/F09/Homework/ The fig. shows the marginal case for two even solutions (3 overall) a=p2 9 7 5 LHS 3 RHS 1 -1 0 -3 -5 0.2 0.4 0.6 0.8 1 1.2 Schrodinger’s Equation Solutions Harmonic Oscillator There are an infinite number of possible states, since the potential is defined to keep going up (of course this is an idealization). Interestingly, the energy levels are evenly spaced in this case: E = hw(n + ½) n = 0, 1, 2, … Schrodinger’s Equation Solutions Harmonic Oscillator n=10 Notice how the quantum probability distribution (blue curve) gets closer to the classical one (dashed line above) as n gets bigger. n=0 Quantum Tunneling in Chemistry McMohan Science 299, 833 (2003) Lecture 22 Potential Barrier: Classical Transmitted Reflected E >Vo <Vo Lecture 22 Potential Barrier: Quantum E <Vo Reflected and Transmitted !! You can watch a demonstration of the optical analog of this on YouTube at: http://www.youtube.com/watch?v=aC-4iSD2aRA Lecture 22 JITT question Identify two examples of phenomena discussed in the text where quantum mechanical tunneling is involved, and cast a vote for which one you would like to see discussed in greater detail in class. •Alpha-decay (18 identified this; 13.5 voted for it) •Tunnel diodes (16 identified this; 4 voted for it) •STM (2 identified this, ½ voted for it) •Internal reflection of light (2 identified it; one voted for it) •Potential wells and barriers (3 identified these; 1 voted for it) Lecture 23 Intro to Radioactivity This chart shows the decay modes of the known nuclides, see the link for an interactive version with lots more information http://www.nndc.bnl.gov/chart/reColor.jsp?newColor=dm Lecture 23 Potential Barrier: Alpha Decay Alpha decay in radioactivity The narrow “squarish” well is provided by the STRONG force, but once the a particle gets far enough away from the other nucleons, it feels the Coulomb repulsion. The a particle ‘leaks’ through the triangle-like barrier. The energy level (above 0) Ea, determines the half-life of the nucleus (i.e. how rapidly the particle tunnels out of the nuclear barrier). Nuclear potential Lecture 23 Potential Barrier: Alpha decay The deeper the “bound” state is below the top of the barrier, the lower will be the kinetic energy of the alpha particle once it gets out, and the slower will be the rate of tunneling (and hence the longer the half-life). Figures from Rohlf “Modern Physics from a to Zo”. Lecture 23 JITT question The STM was the first of what is now a wide assortment of scanning probe microscopies for studying surfaces (Atomic Force, Magnetic Force, Scanning capacitance, Near-field Scanning Optical …). How would you expect the current in an STM to depend on the separation between the tip and the surface? •“decrease as the distance increases” (13 responses) •Inversely as the square of the distance (Coulomb) (2 responses •Exponential decay with distance (4 response) • Other (9 responses) •No response (12) What defines “the surface” in this technique? •“the Surface” (4 responses) •The surface atoms (6 responses) •The “upper electrons” (10 responses) •The boundary of the well (3 response) Lecture 23 Scanning Tunneling Microscope Basic operation of the STM. Piezo-electric actuators move the tip in three directions, typically keeping the current constant and the “surface” is mapped out by monitoring the “z” feedback voltage as the x-y directions are scanned. (the exponential dependence of current on separation is crucial to this). http://www.ieap.uni-kiel.de/surface/ag-kipp/stm/images/stm.jpg Lecture 23 Scanning Tunneling Microscope “High-Resolution” STM measurement of the a surface of Si (7x7 reconstruction of the 111 surface, if you wan to be technical), along with a computer calculation of the electron density predicted for that surface, and a “stick” model of the atomic positions. Taken from the Omictron site: http://www.omicron.de/index2.html?/results/highest_resolution_stm_image_of_si_111_7x7/index.html~Omicron Lecture 23 Scanning Tunneling Microscope Fe atoms arranged by “hand” into a circle on a Cu surface (111 surface to be precise) The ripples inside the “corral” represent the states available to the electrons in the copper in the presence of these extra surface atoms. http://www.almaden.ibm.com/vis/stm/stm.html See also: http://www.youtube.com/watch?v=0wF4f2YadoA Lecture 24 Spherical Polar Coordinates http://en.wikipedia.org/wiki/Spherical_coordinate_system http://en.citizendium.org/wiki/Spherical_polar_coordinates •r defines the sphere •q defines the cone •f defines the plane and • the intersection of the three is the point of interest Lecture 24 Spherical Harmonics http://www.physics.umd.edu/courses/Phys402/AnlageSpring09/spherical_harmonics.gif Lecture 24 Spherical Harmonics http://en.wikipedia.org/wiki/Atomic_orbital Lecture 24 The Hydrogen Atom revisited Major differences between the “QM” hydrogen atom and Bohr’s model (my list): •The electrons do not travel in orbits, but in well defined states (orbitals) that have particular shapes (probability distributions for the electrons, or linear combinations thereof) [9 responses] •The Energy levels are NOT tied directly to the angular momentum. [2 responses] •There are several different states with the same energy in the QM atom [4 answers addressed this but in different words] •Angular momentum is more involved, and more subtle than in the Bohr atom [3 responses] NOTE: the energy levels are (nominally) the same, until we account for subtle effects that lift degeneracy.