* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Welcome to Biochemistry/Endocrinology
Survey
Document related concepts
Magnesium transporter wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Ligand binding assay wikipedia , lookup
Interactome wikipedia , lookup
Biochemistry wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Lipid signaling wikipedia , lookup
Western blot wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Biochemical cascade wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Proteolysis wikipedia , lookup
Paracrine signalling wikipedia , lookup
Transcript
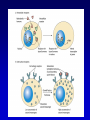
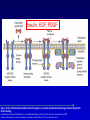
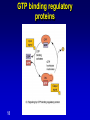
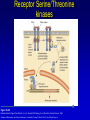
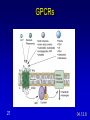
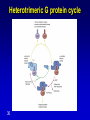
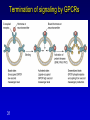
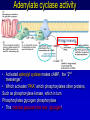
Mechanism of hormone action, signal transduction, 2nd messengers and receptors Reading material Devlin 6e Chapter 13 MAHMOUD A. ALFAQIH BDS PhD Department of Physiology & Biochemistry Jordan University of Science & Technology, School of Medicine Overview of Hormones • Mobile signals secreted by endocrine system for distant cell-cell communication • Occur as four major classes (and several minor ones) – Peptide hormones: tiny “proteins” from 3200 aa’s, water-soluble, active only after cleavage of the targeting “pre-sequence” and the inactive “prohormone” (insulin, glucagon) Continue…………. Overview of Hormones – Amino acid derivative hormones: derived from amino acids, water-soluble, some are neurotransmitters (epinephrine, T3, T4 ) – Eicosanoids: derived from arachidonate, minimally water-soluble, act locally (rather than through the bloodstream), mediate pain, inflammation – Steroid hormones: derived from cholesterol, fat-soluble, are carried to targets by carrier proteins Common Characteristics of Hormones • They occur and function at very low concentrations – 10-6 to 10-12 M • Deliberately unstable – levels rise rapidly upon secretion, but fall fast when it stops • Biochemical response may be very rapid, by altering existing enzyme activities, or slower, where gene expression levels change • Act through two receptor types: cell surface and nuclear • Display remarkable specificity • Operate through the “cascade” principle 6 Two Types of Hormone Receptor • Hormones are present at very low concentration, thus receptor affinity must be very tight • Cell surface receptors bind hormone outside – a conformational change transduces the signal to the inside, resulting in a “second messenger” (e.g. cAMP) • Cytosolic/nuclear receptors bind and retain hormone, migrating to the nucleus as a hormonereceptor complex and then alter gene expression Figure 13.6 Structures of four second -messenger molecules. Textbook of Biochemistry with Clinical Correlations, 7e edited by Thomas M. Devlin © 2011 John Wiley & Sons, Inc . An Overview of Hormone Classes 1. 2. 10 Receptor tyrosine kinase Receptor serine/threonine kinase Figure 13.5 Basic characteristics of receptor subtypes as functionally distinct receptor proteins that bind a common extracellular signaling molecule. Textbook of Biochemistry with Clinical Correlations, 7e edited by Thomas M. Devlin © 2011 John Wiley & Sons, Inc . Mechanism of hormone action, signal transduction, 2nd messengers and receptors ENZYME LINKED RECEPTORS 12 04.12.8 Enzyme linked receptors; Receptor tyrosine kinases (RTKs) Include receptor for insulin, Epidermal Growth factor (EGF), platelet derived growth factor (PDGF) Most are single subunit receptors but some exist as multimeric complexes (Like insulin) Amino end is extracellular (Ligand binding), carboxyl end is intracellular and contains catalytic domain Enzyme linked receptors; Receptor tyrosine kinases (RTKs) Ligand binding induces receptor dimerization and activates catalytic domain Catalytic domain auto-phosphorylates receptor (receptor also functions as an effector protein) Phosphorylated tyrosine residues become anchoring point for adaptor proteins Adaptor proteins have special protein-protein interaction domains called (SH2 for SRC Homology 2) RTKs allow accumulation of many types of tyrosine phosphorylated proteins (secondary effector proteins) Insulin, EGF, PDGF Figure 13.14 Conformational and functional changes in a receptor tyrosine kinase during activation by growth factor binding. Redrawn based on figure from Alberts, B., et al., Molecular Biology of the Cell, 4th ed. New York: Garland Science, 2002. Textbook of Biochemistry with Clinical Correlations, 7e edited by Thomas M. Devlin © 2011 John Wiley & Sons, Inc . Mutations in RTKs can lead to cancer Figure 13.15 Mutated forms of receptor tyrosine kinases as the products of cancer-causing oncogenes. Textbook of Biochemistry with Clinical Correlations, 7e edited by Thomas M. Devlin © 2011 John Wiley & Sons, Inc . GTP binding regulatory proteins They could be functionally coupled to “G protein coupled receptor” (Exist as trimers) They could exist as functionally distinct monomeric proteins They have GTPase activity which allows them to function as “molecular switches” Exchange of GDP with GTP activates the proteins 17 GTP binding regulatory proteins 18 Ras GTPase and MAP Kinases Ras is a small monomeric GTP binding regulatory protein About 30% of all tumors have mutations in Ras GTPase Adaptor proteins can directly bind to phosphorylated RTKs and recruit Ras activating proteins Ras activating proteins enhance GDP exchange with GTP Ras protein can then activate downstream signaling pathways involved in proliferation (MAP kinase cascade) Figure 13.16 Role of the ras GTPase during intracellular signal transduction by an activated receptor tyrosine kinase. Textbook of Biochemistry with Clinical Correlations, 7e edited by Thomas M. Devlin © 2011 John Wiley & Sons, Inc . Ras GTPase and MAPK Figure 13.17 Role of the MAP kinase cascade during intracellular signal transduction by an activated receptor tyrosine kinase. Redrawn based on figure from Alberts, B., et al., Essential Cell Biology, 2nd ed. New York: Garland Science, 2004. Textbook of Biochemistry with Clinical Correlations, 7e edited by Thomas M. Devlin © 2011 John Wiley & Sons, Inc . Signaling by protein phosphorylation 22 Receptor Serine/Threonine Kinase Receptors for transforming growth factor B (TGFB) Important role in cell signaling during tissue differentiation and fetal development Mutations in TGFB mediated signaling pathway leads to cancer 23 Receptor Serine/Threonine kinases Figure 13.18 Redrawn based on figure from Alberts, B., et al., Essential Cell Biology, 2nd ed. New York: Garland Science, 2004. Textbook of Biochemistry with Clinical Correlations, 7e edited by Thomas M. Devlin © 2011 John Wiley & Sons, Inc . Mechanism of hormone action, signal transduction, 2nd messengers and receptors G PROTEIN COUPLED RECEPTORS 25 G protein coupled receptors (GPCRs) GPCRs bind to a diverse range of ligands (proteins, peptides, amino acid derivatives, lipids, nucleotides) Play an important role in endocrine, paracrine, autocrine signaling in all tissues and cell types Sensory proteins just like rhodopsin are GPCRs GPCRs, their ligands and their downstream pathways are targets for many of the currently used drugs GPCRs have a great degree of structural but not sequence similarity 26 GPCRs 27 04.12.8 Heterotrimeric G proteins GPCRs are functionally coupled to trimeric complexes consisting of α, β and γ. α subunit is the largest subunit, hydrophilic and contains GTPase activity Twenty subtypes of α subunit are grouped into subfamilies based on specific downstream action αs Subunit stimulates adenylate cyclase, while αi inhibits it 28 Heterotrimeric G proteins Figure 13.21 Major subclasses of heterotrimeric G proteins that are activated by G by the seventransmembrane domain G-protein-couple receptors. Textbook of Biochemistry with Clinical Correlations, 7e edited by Thomas M. Devlin © 2011 John Wiley & Sons, Inc . Heterotrimeric G protein cycle 30 Termination of signaling by GPCRs 31 Cyclic AMP based signal transduction The use of cAMP as a second messenger is largely limited to GPCRs Cells regulate both the synthesis and the degradation of cAMP cAMP synthesis is catalyzed by adenylate cyclase effector enzyme An integral membrane protein At least six genes that encode different subtypes have been identified Cyclic AMP based signal transduction All subtypes are activated by αs Subunit of G proteins and also inhibited by αi subunit Breakdown of cAMP is catalyzed by a variety of phosphodiesterases that hydrolyze ester bond and lead to the production of AMP Activity of phosphodiesterase is hormonally regulated and is affected by drugs Caffeine inhibits activity of phosphodiesterases leading to accumulation of cAMP Breakdown of cAMP 34 Adenylate cyclase activity Protein kinase A • Activated adenylyl cyclase makes cAMP, the “2nd messenger”, • Which activates “PKA” which phosphorylates other proteins, Such as phosphorylase kinase, which in turn Phosphorylates glycogen phosphorylase • This mobilize glucose from liver glycogen! Intracellular signaling mechanism of cAMP 36 Turn off of the signal: 1. Ga hydrolyzes GTP to GDP + Pi. (GTPase). The presence of GDP on Ga causes it to rebind to the inhibitory bg complex. Adenylate Cyclase is no longer activated. 2. Phosphodiesterase catalyzes hydrolysis of cAMP AMP. Turn off of the signal (cont.): 3. Receptor desensitization occurs. This process varies with the hormone. Some receptors are phosphorylated via specific receptor kinases. The phosphorylated receptor may then bind to a protein arrestin, that promotes removal of the receptor from the membrane by clathrin-mediated endocytosis. 4. Protein Phosphatase catalyzes removal by hydrolysis of phosphates that were attached to proteins via Protein Kinase A. Signal amplification is an important feature of signal cascades: One hormone molecule can lead to formation of many cAMP molecules. Each catalytic subunit of Protein Kinase A catalyzes phosphorylation of many proteins during the life-time of the cAMP. Activation of PKA heterotetramer (inactive) dimer active monomers Cyclic GMP based signal transduction Levels of cGMP are regulated by a balance between guanylate cyclase and cGMP phosphodiesterase Guanylate cyclase could be either membrane bound or a free cytosolic protein (soluble) Membrane bound guanylate cyclase could be activated by ANF (atrial natriuretic factor) Soluble Guanylate cyclase is activated by NO (nitric oxide) 41 Cyclic GMP based signal transduction Activation of guanylate cyclase leads to relaxation of vascular smooth cells through activation of cGMP sensitive protein kinases Mechanism: 1. Attenuation of calcium release from sarcoplasmic reticulum 2. Activation of myosin light chain phosphatases 3. Activation of potassium channels leading to hyperpolarization of myocyte membrane 42 Signaling mechanism of cGMP 43