* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download File
Survey
Document related concepts
Rosetta@home wikipedia , lookup
Protein design wikipedia , lookup
Structural alignment wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Circular dichroism wikipedia , lookup
Protein purification wikipedia , lookup
Protein domain wikipedia , lookup
Homology modeling wikipedia , lookup
Protein folding wikipedia , lookup
Protein moonlighting wikipedia , lookup
List of types of proteins wikipedia , lookup
Western blot wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Alpha helix wikipedia , lookup
Transcript
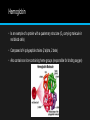
2.4 Proteins Proteins have a very wide range of functions in living organisms. Amino Acids • Proteins are composed of long chains of recurring monomers called a.a. • There are 20 different a.a. which are universal to all living organisms. • a.a. all share a common basic structure, with a central carbon atom bound to: • Amine group (NH2) • Carboxylic group (COOH) • Hydrogen atom (H) • Variable side chain (R) • Amino acids are joined together on the ribosome to form long chains called polypeptides, which makes up proteins. • Each type of a.a. differs in the composition of the variable side chain (R). • Different side chains will have distinct chemical properties (charged, nonpolar). • The protein will fold and function differently according to its specific position within the polypeptide chain. Formation of a Dipeptide • Amino acids can be covalently joined together in a condensation reaction to form a dipeptide and water. • Polypeptides (long chain of covalently bonded a.a.) chains can be broken down via hydrolysis reactions, which requires water to reverse the process. • Peptide bonds are formed between the amine and carboxylic acid groups of adjacent amino acids. • The amine group loses a hydrogen atom (H) and the carboxylic acid loses a hydroxyl (OH) – this forms water (H2O) Protein Structures • The order of the amino acid sequence is called the primary sequence and determines the way the chain will fold. • Different amino acid sequences will fold into different configurations due to the chemical properties of the variable side chains. • Amino acid sequences will commonly fold into two stable configurations, called secondary structures. • Alpha helices occur when the amino acid sequence folds into a coil/spiral arrangement • Beta-pleated sheets occur when the amino acid sequences adopts a directionally-oriented staggered strand conformation. SEcondary Structures • Both a-helices and B-pleated sheets result from hydrogen bonds forming between non-adjacent amine and carboxyl groups. Tertiary Structures • The overall 3D configuration of the protein is referred to as the tertiary structure of the protein. • The interaction between the variable side chains is what determines the structure. • These interactions may include hydrogen bonds, disulphide bridges, ionic interactions, polar associations, etc. • The affinity or repulsion of side chains will affect the overall shape of the polypeptide chain and are determined by the position of specific amino acids within a sequence. • Hence, the order of the a.a. sequence (primary structure) determines all subsequent levels of protein folding. Quaternary Structure • Certain proteins possess a fourth level of structural organization, quaternary structure. • Quaternary structures are found in proteins that consist of more than one polypeptide chain linked together. • Alternatively, proteins may have a quaternary structure of they include organic prosthetic groups as part of their structure. • Not all proteins will have a quaternary structure- many proteins consist of a single polypeptide chain. Some examples of proteins and their functions….yes you need to know all of these!!! Protein Function Rubisco The short-hand name for the enzyme that catalyzes the first reaction of the carbon-fixing reactions of photosynthesis Insulin A protein hormone produced by the pancreas that results in a decrease of blood sugar levels and an increase of sugar inside body cells. Immunoglobulin Another name for an antibody that recongnizes an antigen as part of the immune response. Rhodopsin A pigment found in the retina of the eye that is particularly useful in low light conditions Collagen The main protein component of connective tissue, which is abundant in skin, tendons, and ligaments. Spider silk A fibrous protein spun by spiders for making webs, drop lines, nest building and other uses. Hemoglobin • Is an example of a protein with a quaternary structure (O2 carrying molecule in red blood cells) • Composed of 4 polypeptide chains (2 alpha, 2 beta) • Also contains an iron-containing heme groups (responsible for binding oxygen) Denaturation of proteins • Denaturation is a structural change in a protein that results in the loss (usually permanent) of its biological properties. • The way it folds determines its function, any change will alter its activity. • Temperature and pH are two conditions that can cause denaturation. Unique Proteome • Each organisms is genetically different from other organisms. • Sexual reproduction ensures this as well. • Specific DNA sequence, unique to an individual is called their genome. • DNA codes for proteins, each person is capable of synthesizing their own set of proteins, so they have their own unique proteome as well as genome. Temperature • High levels of thermal energy may disrupt the hydrogen bonds that hold the protein together. • As the bonds are broken, the protein will begin to unfold and lose its capacity to function as intended. • Temperatures at which proteins denature may vary, but most human proteins function optimally at body temperature (37 C) pH • Amino acids are neutral molecule possessing both negatively (COO-) and positively (NH3+) charged regions. • Changing the pH will alter the charge of the protein, which in turn will alter protein solubility and overall shape. • All proteins have an optimal pH which is dependent on the environment in which it functions.