* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Why should we study B12 and Folate? Deficiencies in both are still
Human digestive system wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Point mutation wikipedia , lookup
Genetic code wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Butyric acid wikipedia , lookup
Proteolysis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Metalloprotein wikipedia , lookup
Plant nutrition wikipedia , lookup
Biochemistry wikipedia , lookup
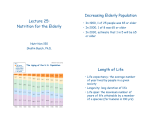
B12 and Folate 10/17/2008 4:39:00 PM Why should we study B12 and Folate? Deficiencies in both are still somewhat common. Deficiencies can cause major health problems and birth defects They present very similarly. Learning how to differentiate is very important for treatment Vitamin B12 Cobalamin o Corrin Ring with Cobalt in the center o Dimethylbenzimidizole ribonucleotide (ribonucleotide-esque) o R-Group Cyano (-CN) is found in cyanocobalamin. Cyano- is the most common form as it is the form that is marketed Hydroxyl (-OH) is found in meat and is the most commonly ingested Methyl (-CH3) Methylcobalamin is the form found in Methionine synthase 5’ deoxyadenosyl is the coenzyme for methylmalonyl CoA Mutase o Cobalt interacts with 6 other atoms Four nitrogen from the corrin ring 1 Nitrogen from the benzimidizole 1 atom from the R group Why is B12 required? o Two enzymes Methionine Synthase Methylcobalamin acts a methyl donor Methyl-THF is required as it regenerates the methylcobalamin Methionine Cycle Methionine is converted to S adenosyl Methionine, which is a methyl donor. Product is Homocysteine Homocysteine is converted back to methionine by methionine synthase Deficiency leads Homocysteinuria/Homocysteinemia o Independent risk factor for CAD Effective folate deficiency as Methyl-THF cannot be converted to THF so it can be used in other reactions o Folate is transported as methyl-THF and only methionine synthase can use methyl-THF Methylmalonyl CoA Mutase Converts Methylmalonyl CoA that is generated during branched amino acid metabolism (among other things) B12 is stored in the liver o Supply is sufficient for 3-5 years o RDA of 2 µg per day o Pernicious Anemia—Lack of B12 due to lack of intrinsic factor (result of deteriorating stomach lining) o B12 is only made by microorganisms, but is richest in meat. B12 travels up the food chain B12 Uptake o B12 is bound to its R group with the other proteins that it’s related to. o Stomach acid degrades the protein and releases B12. R Binder protein made in the salivary glands will then bind to the R Group and transport it to the small intestine. o In the Small intestine, proteases chew up the R binder protein and the B12 is released. It will then bind to intrinsic factor and be marked for endocytosis. o B12 is transported in the blood by Transcobalamin I and stored in the liver as Transcobalamin II Folate Structure o Three major parts Pterin Ring P-aminobenzoic acid (PABA) glutamate o Three major variables Polyglutaminylation Can have many glutamines added to the carboxyl of the glutamine Different reduction states Different amounts of hydrogen Three reduction states: Folate, DHF, THF Addition of 1 carbon units to the ring N5 and/or N10 involved in binding Allows folate to add 1 carbon units to compounds Carbon substituents on folate are interchangeable with the exception of methyl Uptake o Folate is found in leafy vegetables o Transported as methyl THF o Cannot be used for other reactions until converted to THF by methionine synthase o RDA 200 µg per day (400-800 µg per day for pregnant women) o 5-10 mg stored in liver Sufficient for 3-6 months Importance (if deficient this happens) o Homocysteinemia/Homocysteinuria is caused by the inability to regenerate methylcobalamin o Neural Tube Defects in developing fetus due to lack of nucleotide synthesis o Megaloblastic Anemia due to lack of nucleotide synthesis o Most symptoms of folate deficiency are seen in B12 deficiency because if there is no action of methionine synthase, there will be no available THF. Sulfa Drugs inhibit folate synthesis in bacteria by substituting analogs of p-aminobenzanoic acid. They only affect bacteria because we don’t synthesize our own folate. Diagnosis Test for serum folate, serum B12 and methylmalonic acid. o Methylmalonic acid will be high in B12 deficient persons but normal in folate deficient persons. Objectives 10/17/2008 4:39:00 PM 1. Describe the general structure of Vitamin B12, the forms in which it is normally ingested and the active coenzyme forms. a. Corrin Ring b. Benzimidizole c. R group i. Ingested form is usually hydroxycobalamin ii. Vitamin form is cyanocobalamin iii. Methionine Synthase form is methylcobalamin iv. Methylmalonyl CoA Mutase form is 5’ deoxyadenosylcobalamin 2. Describe the role of intrinsic factor and the transcobalamins in the uptake and storage of Vitamin B12 a. Intrinsic factor is necessary to signal for B12 endocytosis. Transcobalamins are necessary for transport in the blood (transcobalamin I) and storage in the liver (transcobalamin II) 3. Name and write the two enzyme catalyzed reactions in humans that require cobalamin a. Methionine Synthase b. Methylmalonyl CoA Mutase 4. Compare B12 and folate daily requirements, acquisition and storage in the body. a. B12—2 µg per day stored in the liver for 3-5 years before deficiency 5. Describe the metabolic effects of B12 deficiency. a. B12 Deficiency will decrease nucleotide synthesis and amino acid metabolism. The former of the two is way worse. 6. Explain how B12 and folate deficiencies can induce megaloblastic anemia, and how to distinguish between the two. a. Megaloblastic anemia is caused by deficiencies in DNA synthesis needed or dividing cells. B12 and Folate deficiencies can be differentiated by testing serum levels of both B12, Folate and methylmalonyl CoA. If the last compound is elevated, you know the problem is with B12. If it is normal, the problem is Folate 7. Recognize the chemical structures and different active forms of the folate coenzymes a. Pterin Ring b. p-aminobenzoic acid (PABA) c. Glutamic Acid 8. List 5 reactions that require folate coenzymes a. Methylation of Cobalamin b. Addition of the C8 carbon on purine synthesis c. Addition of the C2 carbon in purine synthesis d. Methylation of uracil to make thymidine e. Serine-glycine hydroxymethyl transferase (it makes methyl THF in reverse or uses methyl THF to make serine) 9. Describe the folate trap hypothesis and how it explains some of the symptoms of B12 deficiency. a. If there is a B12 deficiency, there will not be any methyl THF converstion to THF so the cell cannot make any of the other one carbon THF molecules. This is called functional THF deficiency. Lack of B12 causes a lack of functional Folate 10. Explain the importance of folate polyglutamylation a. Polyglutamylation causes increased retention of Folate in the cell. 11. Describe the mechanism of inhibition of bacterial growth by sulfonamides. a. Sulfonamides imitate the p-aminobenzanoic acid molecules necessary for folate production. It mucks up the synthetic pathway and causes folate deficiency. 10/17/2008 4:39:00 PM