* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cases - Google Sites
Survey
Document related concepts
Transcript
Pediatric Rotation
(8/10/2006)
Morning Reports Schedule
Date
PRH
KAUH 4th
Pediatricia Case
08/10/2006
Wail
09/10/2006
Azhar / wail
10/10/2006
Waddah
11/10/2006
12/10/2006
Khasaw
15/10/2006
16/10/2006
Wail / Azhar
17/10/2006
Jahed
18/10/2006
Samah
19/10/2006
Suleiman
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Pediatricia Case
Khasawneh
Wail
hala
Wail
Samah
Khasa
Waddah
Azhar
Miqdady
10/22 10/28
29/10/2006
Miqdady
30/10/2006
Wail / Azhar
31/10/2006
Waddah
01/11/2006
Hala
02/11/2006
05/11/2006
06/11/2006
Wail / Azhar
07/11/2006
08/11/2006
Hala / samah
09/11/2006
Suleiman
12/11/2006
13/11/2006
Wail / Azhar
14/11/2006
Waddah /
Jahed
15/11/2006
16/11/2006
Suleiman
19/11/2006
Miqdqdy
20/11/2006
Wail
21/11/2006
22/11/2006
Hala / samah
23/11/2006
Suleiman
26/11/2006
27/11/2006
Azhar
28/11/2006
29/11/2006
Waddah /
Jahed
Hala / samah
30/11/2006
Suleiman
03/12/2006
Miqdady
04/12/2006
Azhar
05/12/2006
Waddah
06/12/2006
Samah / hala
07/12/2006
10/12/2006
Miqdady
Open
Open
Open
Open
Open
Khasa
Open
Open
Open
Open
Open
Samah
Open
Open
Open
Wail
Hala
Wail
Samah
Jahed
suleiman
waddah
azhar
Khasa
Mafraq 4th
Pediatrician Case
KAUH 6th
Pediatrician
Case
Hala / Jahed
Open
Open
Open
Open
Open
Neonatal sepsis Mafraq
Meningitis
Mafraq
GERD
Mafraq
Pulmonary infections Mafraq
Croup & epiglttitis Hala / Waddah
Nephrotic
Jahed / Azhar
Febrile seizures
Shock
Mafraq
Mafraq
Respiratory distress Mafraq
Cerebral palsy
Mafraq
Malabsorption
Mafraq
عطلة عيد الفطر اسبوع
Nephritis
UTI
Miqdady / Jahed
Failure to thrive
Samah / Suleiman
Hepatitis
Asthma
Qtaish / Miqdady
Neonatal sepsis Mafraq
GERD
Mafraq
Meningitis
Mafraq
Pulmonary infections Mafraq
Nephrotic
Mafraq
Open
Hala / Jahed
Nephrotic
Azhar / Wail
Febrile seizures
Malabsorption
Mafraq
Mafraq
Respiratory distress Mafraq
Cerebral palsy
Mafraq
Shock
Mafraq
Nephritis
UTI
Miqdady / Hala
Failure to thrive
Samah / Suleiman
Hepatitis
Asthma
Miqdady / Qtaish
Pulmonary infections
Open
Hala / Jahed
Mafraq
Mafraq
Mafraq
AGE
Jahed
Neonatal jaundice Qtaish / Miqdady
Waddah
Wail / Azhar
Croup & epiglttitis Waddah / Khasa
AGE
Samah
Neonatal jaundice Qtaish / Miqdady
Waddah / Khasa
Azhar / Wail
Hala
GERD
Wail
Meningitis
Open
Open
Khasa
Neonatal sepsis Mafraq
Nephritis
Mafraq
Neonatal jaundice Qtaish / Samah
Nephrotic
Wail / Azhar
Open
Open
Open
Open
Open
Samah
Febrile seizures
Malabsorption
Shock
Mafraq
Mafraq
Mafraq
Respiratory distress Mafraq
Cerebral palsy
Mafraq
Nephritis
UTI
Hala / jahed
Failure to thrive
Suleiman / samah
Hepatitis
Asthma
Qtaish / miqdady
Open
Open
Open
Wail
Pulmonary infections
Mafraq
Neonatal sepsis Mafraq
Meningitis
Mafraq
Open
Hala / Jahed
Open
Open
Miqdady
GERD
Nephrotic
Neonatal jaundice Qtaish / miqdady
Open
Open
Open
Open
Open
Samah
Open
Wail
Jahed
Suleiman
Qtaish
Khasaw
Azhar
Waddah
Wail
Jahed
Khasaw
Jahed
Azhar
Miqdady
Mafraq
Mafraq
Croup & epiglttitis Khasa / waddah
AGE
samah / Suleiman
Khasa / Azhar
Wail
Croup & epiglttitis Wail / Hala
AGE
Suleiman / Samah
Nephrotic
Wail / Azhar
Mafraq
Mafraq
Respiratory distress Mafraq
Cerebral palsy
Mafraq
Malabsorption
Mafraq
Nephritis
UTI
Hala / Jahed
Failure to thrive
Suleiman / Samah
Hepatitis
Asthma
Qtaish / Miqdady
Pulmonary infections
Open
Hala / Jahed
Febrile seizures
Shock
Mafraq
Waddah
Suleiman / Azhar
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
Open
11/12/2006
Wail
12/12/2006
Jahed
13/12/2006
Hala / samah
14/12/2006
17/12/2006
Miqdady
18/12/2006
Wail / Azhar
19/12/2006
Jahed
20/12/2006
Hala / samah
21/12/2006
Suleiman
Open
Open
Hala
Open
Open
Miqdady
Open
Open
Open
Open
Open
Khasa
Wail
Jahed
Suleiman
Neonatal sepsis Mafraq
Meningitis
Mafraq
GERD
UTI
Mafraq
Mafraq
Shock
Febrile seizures
Mafraq
Mafraq
Mafraq
Mafraq
Mafraq
Azhar
Open
Azhar
Cerebral palsy
Malabsorption
Miqdady
Croup & epiglttitis Khasa / waddah
Suleiman / samah/
Waddah
Neonatal jaundice Qtaish / Suleiman
AGE
Nephrotic
Wail / Khasa
Nephritis
UTI
Hala / Jahed
Failure to thrive
Suleiman / samah
Hepatitis
Asthma
Qtaish / miqdady
Khasa / waddah
Wail
Open
Open
Open
Open
Open
Open
Open
Open
Open
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
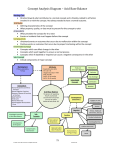
Croup and Epiglottitis
Case
This is a 20 month old male who presents to the emergency department with a chief
complaint of cough. Two days ago he developed rhinorrhea, fever, a hoarse cry and a
progressively worsening, harsh, "barky," cough. Today he developed a "whistling"
sound when he breathes, so his parents brought him to the emergency department.
His past medical history is unremarkable. His 6 year old brother also has cold
symptoms.
Exam: VS T 37.5, P 140, R 36, BP 90/64, oxygen saturation 96% in room air. He is
alert, with good eye contact, in mild respiratory distress. He has a dry barking cough
and a hoarse cry. He has some clear mucus rhinorrhea but no nasal flaring. His
pharynx is slightly injected, but there is no enlargement or asymmetry. His heart is
regular without murmurs. His lung exam shows good aeration and slight inspiratory
stridor at rest. He has very slight subcostal retractions. No wheeze or rhonchi are
noted. His abdomen is flat, soft, and non-tender. His extremities are warm and pink
with good perfusion.
He is treated with nebulized racemic epinephrine and his coughing subsides and his
stridor resolves. A lateral neck X-ray reveals no prevertebral soft tissue widening or
evidence of epiglottitis. The subglottic region is mildly narrowed. He is treated with
oral dexamethasone. He is discharged home after one hour of monitoring and his
parents were instructed to treat him with humidified mist therapy.
Croup, which is derived from an Anglo-Saxon word meaning "to cry out", is a
common respiratory illness in childhood. Croup is also known as laryngotracheitis
and laryngotracheobronchitis (LTB). These terms will be used interchangeably in this
chapter. The diagnosis describes a disease with some degree of laryngeal
inflammation; resulting in hoarseness, a barking cough and varying degrees of
respiratory distress over time. There are different etiologies encompassed in the
diagnosis of croup, but the most common cause is viral, and this will be the focus of
this chapter. The entity known as spasmodic croup is not easily distinguished from
viral croup except that spasmodic croup has a greater tendency to recur. The
treatment and evaluation are similar. When evaluating a child with croup, it is
important to rule out epiglottitis, so this will be discussed as well.
Croup occurs most commonly between the ages of 1 and 6 years, with a peak
incidence being around 18 months of age and the majority of cases below 3 years of
age. It is more common in boys than girls. In temperate climates, it is most common
during the late fall and winter, although cases can occur throughout the year.
Parainfluenza viruses are the most frequent cause of croup, accounting for more than
60% of cases. Less frequently associated with croup are influenza A and B,
respiratory syncytial virus, adenovirus and measles. Bacterial superinfection can
occur in cases of laryngotracheobronchitis and laryngotracheobronchopneumonitis.
Like most respiratory infections, the initial site of infection is thought to be the
nasopharynx with subsequent spread to the larynx and trachea. The respiratory
epithelium becomes diffusely inflamed and edematous, resulting in airway narrowing
and stridor. Reduced mobility of the vocal cords results in a hoarse voice or cry.
Laryngotracheitis generally starts with several days of rhinorrhea, pharyngitis, lowgrade fevers and a mild cough. Over the next 12 to 48 hours, a progressively
worsening "barky" cough, hoarseness and inspiratory stridor are noted, secondary to
some degree of upper airway obstruction and laryngeal inflammation. The speed of
progression and degree of airway obstruction can vary widely. The onset is often
rapid and typically in the early morning hours (e.g., 2:00 am). Croup symptoms
appear to subside during the day (possibly because of positioning), only to recur the
following night. Thus, a child with significant stridor presenting during daylight, may
be more seriously affected. On examination, the child will be noted to have coryza, a
hoarse voice, and varying degrees of pharyngeal inflammation, tachypnea, and stridor.
More severe cases may involve nasal flaring, moderate tachypnea, retractions and
cyanosis. Some children with croup may not be able to maintain adequate oral intake
of fluids. Alveolar gas exchange is usually normal, with hypoxia seen only in severe
cases. Symptoms of croup usually normalize over 3-7 days, although in severely
affected children, this may take 7-14 days.
The diagnosis is usually made on clinical grounds. Laboratory studies add little to the
diagnosis of croup if bacterial infection is not suspected. White blood cell counts may
be elevated above 10,000 with a predominance of polymorphonuclear cells. White
blood cell counts greater than 20,0000 with bandemia may suggest bacterial
superinfection. Chest radiographs may show subglottic narrowing (in 50% of
children with croup), but this can also be seen in normal patients. Lateral neck
radiographs are often obtained, not as much to confirm the diagnosis of croup, but to
rule out other causes of stridor such as soft tissue densities in the trachea, a
retropharyngeal abscess and epiglottitis.
The most important diagnostic consideration is distinguishing acute epiglottitis from
acute laryngotracheitis. Epiglottitis describes a bacterial infection of the epiglottis. It
is most commonly caused by H. influenzae type B, and occasionally by S.
pneumoniae and group A Streptococcus. The prevalence of epiglottitis has decreased
markedly (almost non-existent) since the widespread use of H. influenzae B vaccine.
The peak incidence of epiglottitis is between the ages of 3 and 7 years, with cases
described in infants and adults as well. It occurs throughout the year, but is more
common in winter months. Children with epiglottitis do NOT have a "croupy" cough.
They appear more toxic, stridorous, apprehensive, have higher fever (e.g., 40 degrees
C, 104 degrees F) and will often be drooling. Patients will often be tachycardic and
tachypneic. The child with epiglottitis may prefer to adopt a position of sitting up,
leaning forward, with their chin pushed forward and they may refuse to lie down.
They will have a very inflamed, swollen epiglottis. Lateral neck radiographs may be
helpful in making the diagnosis. X-rays are usually deferred if this diagnosis is
suspected, owing to the critical clinical condition of the patient. The three
characteristic findings on lateral neck X-ray are: a swollen epiglottis (thumb sign),
thickened aryepiglottic folds and obliteration of the vallecula (pre-epiglottic space).
Lab work is usually not done, but if done generally reveals elevated white blood cell
counts with a left shift and blood cultures are positive in 80-90% of cases.
Other entities on the differential include bacterial laryngotracheobronchitis and
laryngotracheobronchopneumonitis, which will have signs of lower respiratory
involvement such as, wheezing and/or changes on chest x-ray. Often they will have
hypoxia secondary to the lower airway disease. Retropharyngeal or peritonsillar
abscess can cause upper airway obstruction, with soft tissue swelling evident on
lateral neck x-ray (widening of the prevertebral soft tissue) or physical exam
respectively. These children will often have high fever, drooling and be more toxic in
appearance. Laryngitis can be seen in older children and adults, with a similar
prodrome and cough, but lacking the inspiratory stridor. Foreign body aspiration
should be considered in cases of sudden onset stridor without cough or fever. Acute
angioneurotic edema, can cause acute swelling of the upper airway, but usually
presents with external evidence of swelling of the face and neck. Laryngeal
diphtheria (sometimes presents with a croup like syndrome known as membranous
croup), although rare, should be considered and is another reason to assess the
immunization record.
Once the diagnosis of croup is made, mist therapy, corticosteroids and epinephrine are
the usual treatments. Since croup is chiefly viral in etiology, antibiotics play no role.
Historically, mist therapy has been the mainstay of croup therapy, yet in small empiric
trials, mist therapy has shown little benefit. Mist therapy (warm or cool) is thought to
reduce the severity of croup by moistening the mucosa and reducing the viscosity of
exudates, making coughing more productive. For patients with mild symptoms, mist
therapy may be all that is required and can be provided at home.
For more severe cases, further intervention may be required. Oxygen should be
provided to patients with hypoxemia. Racemic epinephrine, given by nebulizer, is
thought to stimulate alpha-adrenergic receptors with subsequent constriction of
arterioles and decreased laryngeal edema. Nebulized epinephrine may have marked
effect to decrease inspiratory stridor and the work of breathing. Adverse effects
include tachycardia and hypertension. The effects of this medication last less than
two hours and children need to be monitored (not necessarily in the hospital) serially
for the return of symptoms. Racemic epinephrine is a mixture of 50% biologically
active epinephrine and 50% inactive epinephrine. The usual dose is 0.5cc of the
2.25% concentration diluted with 2cc of saline. 0.5cc of the 2.25% is equal to 11 mg
of racemic epinephrine or 5.5 mg of plain epinephrine (0.5 cc of 2.25 gm/100cc = 11
mg). Thus, 5cc of 1:1000 epinephrine solution is pharmacologically similar and can
also be used for inhalation therapy with a nebulizer if racemic epinephrine is not
available.
Corticosteroids provide benefit for children with viral croup by reducing the severity
and shortening the course of the symptoms. Dexamethasone is the most commonly
used, with the dose being 0.6 mg/kg (maximum 10 mg) by mouth or intramuscularly.
Clinical improvement from corticosteroids is usually not apparent until 6 hours after
treatment. More recent studies have shown high dose nebulized budesonide to be as
effective as dexamethasone, with more rapid onset of effect.
Endotracheal intubation is reserved for children with severe symptoms who do not
respond to the previous therapies. This decision should be based on criteria such as
hypercarbia, impending respiratory failure and changes in mental status.
If epiglottitis is suspected, the most serious complication is sudden airway
obstruction. Because of this, airway management becomes the most important
consideration. Visualization of the epiglottis should not be attempted, unless clinical
suspicion is low or respiratory failure occurs. Assistance from a surgeon, intensivist,
anesthesiologist, etc. (more than one is better), should be sought immediately since
patients with epiglottitis may arrest at any time. Intubation is difficult so preparation
should be made for intubation or tracheostomy. If the child is stable, it may be
possible to start at intravenous line and obtain radiographic studies. Once the airway
is secure, IV antibiotic therapy with either ceftriaxone or cefotaxime should be
initiated. In the event of a respiratory arrest, mask ventilation with 100% FiO2 should
be attempted using a two-person technique with one person ensuring a tight mask fit
and the other squeezing the ventilation bag hard enough to drive air through the
narrowed airway. Placing the patient prone (instead of the usual supine position) may
improve ventilation by utilizing gravity to lift the epiglottitis off the larynx.
Most children with croup do extremely well and do not require hospitalization. Most
children can be discharged from the emergency department after receiving
dexamethasone and epinephrine therapy if they have no stridor at rest, normal color,
aeration and level of consciousness and have been monitored for a period of time. 3-4
hours of observation is often recommended, but this is rarely followed in actual
practice since most families are reluctant to remain in the emergency department
during the early morning hours if their child is now sleeping comfortably.
Questions
1. Which of the following viruses are most commonly associated with viral croup?
a. Adenovirus.
b. Human papilloma virus
c. Varicella virus
d. Parainfluenza viruses
e. RSV
2. True/False: An acutely ill child presents to the emergency department with the
signs and symptoms of acute epiglottitis. The diagnosis should be confirmed with
direct visualization of the epiglottis?
3. Which of the following is/are true?
a. There is good evidence from randomized controlled trials that mist therapy
is effective for the treatment of croup.
b. Antibiotics are indicated in the treatment of croup.
c. Nebulized albuterol is effective in the treatment of croup.
d. Dexamethasone has been shown to be effective in the treatment of croup.
4. Which of the following is/are true?
a. Croup affects more girls than boys.
b. Croup shows no seasonal prevalence.
c. Most cases occur in teenagers.
d. It is a common respiratory infection in children.
5. True/False: Once a child with croup has been given corticosteroid treatment and
racemic epinephrine, they may safely be discharged home after 20-30 minutes of
monitoring.
Answers to questions
1. d.
2. False. Routine airway visualization is stressful and may precipitate
respiratory arrest. If epiglottitis is unlikely, then airway visualization appears to be
safe. In the event of respiratory arrest, laryngoscopy will be necessary for tracheal
intubation.
3. d is the best answer. c is also correct in that nebulized albuterol does have
some efficacy in croup, but nebulized epinephrine is better.
4. d.
5. Most textbooks would suggest that this is false in that a longer observation
period is generally recommended. However, most patients are low risk and can be
discharged soon after dexamethasone and epinephrine are administered. Severe
patients or those who do not respond as well should be observed for longer periods of
time.
References
1. Malhotra A, Krilov LR. Viral Croup. Pediatr Rev 2001;22(1):5-12.
2. Cherry JD. Chapter 22 - Croup. In: Feigin RD, Cherry JD (eds).
Textbook of Pediatric Infectious Disease, 4th edition. 1998, Philadelphia: WB
Saunders, pp. 228-241.
3. Klassen TP. Croup: A Current Perspective. Pediatr Clinics North Am
1999;46(6):1167-1178.
4. Cherry JD. Chapter 21 - Epiglottitis. In: Feigin RD, Cherry JD (eds).
Textbook of Pediatric Infectious Disease, 4th edition. 1998, Philadelphia: WB
Saunders, pp. 228-241.
5. Fleisher GR. Chapter 84 - Infectious Disease Emergencies. In: Fleisher
GR, Ludwig S (eds). Textbook of Pediatric Emergency Medicine, fourth edition.
2000, Baltimore: William & Wilkins, pp. 745-750.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
Pulmonary infection:
A previously healthy 4 year old boy is brought to an urgent care center by his mother
for difficulty breathing for one day. Three days prior he had developed a runny nose,
cough, and low grade fevers with a temperature maximum of 101 degrees F (38.3
degrees C). He continued to take liquids well, but his solid intake has decreased. His
temperature this morning was 103 degrees F (39.4 degrees C) and he was breathing
fast and working hard to breathe. He does not have any ill contacts. He has never
been hospitalized or had any surgeries. He was born at term without any
complications. He is not taking medications other than acetaminophen. His
immunizations are up to date for his age (except he had not received the
pneumococcal conjugate vaccine). His parents and 10 year old sister are healthy and
the remainder of his family history is non-contributory. There are no smokers in the
household, and he has not traveled recently. He does not have a history of choking or
vomiting. He has not had frequent ear or skin infections. He does not have a history
of foul-smelling stools.
Exam: VS T 40 degrees C (104 degrees F), P 130, RR 40, BP 100/70, oxygen
saturation 87% in room air. His height and weight are in the 50th percentile for his
age. He is awake and alert, in moderate distress. His conjunctiva and TMs are
normal. His nasal mucosa is erythematous with yellowish discharge. His lips and
mucous membranes are dry. His neck is supple, with several small anterior cervical
lymph nodes.
Lungs:
Moderate subcostal, intercostal, and supraclavicular
retractions, symmetric expansion, dullness to percussion at the right base, increased
vocal fremitus over the right base, decreased air entry over right lower lobe with
crackles, no wheezes. Heart: Tachycardia, regular rhythm without murmur. Pulses
are 2+, and capillary refill time is 3 seconds. His abdomen, skin, and neurological
examinations are unremarkable.
CBC WBC 20,000, 70% segs, 11% bands, 15% lymphs, 3% monos, 1% eos.
Hemoglobin 12.4, platelet count 280,000. Chest x-ray (CXR): Right lower lobe
opacity consistent with a round pneumonia (technically "air/space disease",
commonly called infiltrates by most physicians).
Because of the hypoxia, he is given supplemental oxygen (with subsequent
improvement in oxygen saturation), as hospitalization arrangements are made. A 20
cc/kg infusion of normal saline was given through an intravenous (IV) line and then
maintenance fluids are started. A blood culture is obtained and he is started on IV
cefuroxime. He improves over the next day. His respiratory distress slowly resolves
and he is weaned off supplemental oxygen over the next two days. His blood culture
shows no growth. He is discharged home on high dose amoxicillin for a total of 10
days of therapy. His discharge diagnosis is probably pneumococcal pneumonia.
Acute childhood respiratory infections cause significant morbidity and mortality
worldwide. Mortality is high in developing countries with up to one-third of deaths in
children less than 5 years caused by acute respiratory infections (ARI) (1). The
disparity in mortality is due to the severity of infection (perhaps due to differences in
nutrition, overall health, immunization practices, and medical care availability) since
the incidence of acute respiratory infections is similar between developed and
developing countries with infants experiencing about 4-8 episodes per year (1). In the
US, mortality from ARIs has declined since 1968 (1,2).
There are 2 basic classification systems used for acute respiratory tract infections: the
case-management classification system used by the World Health Organization
(WHO) and the "traditional" clinical classification system (1). The case management
system divides ARIs by symptoms (i.e., stridor, wheezing, and no wheezing) and their
severity (i.e., mild, moderate, severe, and very severe). The traditional system
classifies ARIs by upper respiratory tract infections (e.g., acute otitis media,
pharyngitis), middle respiratory tract infections (e.g., croup and epiglottitis), and
lower respiratory tract infections (e.g., bronchiolitis, bronchitis, pneumonia).
Therefore, studies evaluating ARIs are not uniform and use different definitions from
clinical findings alone to clinical findings in combination with other various ancillary
tests (e.g., chest radiography).
Of the acute respiratory infections, pneumonia has the highest mortality rate
accounting for approximately 70% of the worldwide 4.5 million deaths from acute
respiratory infections (4). Although mortality from pneumonia in children in the
United States has declined by 97% between 1939 and 1996 (5), pneumonia continues
to be a leading cause of morbidity in children. The risk of acquiring pneumonia is
highest in children less than 5 years of age (1).
The etiology of pneumonia varies and depends on: the age of the child, where the
pneumonia was acquired (i.e., community vs. nosocomial), local epidemiology (e.g.,
influenza epidemics), host factors (e.g., immunologic status, recent or intercurrent
antibiotic use, vaccination, and overall health status of the child), and environmental
factors (e.g., travel, season of the year, daycare, or crowded living conditions) (4,6-9).
Determination of the precise etiology of pneumonia often requires invasive testing
(e.g., lung biopsy), and therefore, this is done infrequently. Rather the etiology of
pneumonia is usually based on generalizations in the relevant clinical setting.
In the neonatal period, the most common cause of bacterial pneumonia is group B
beta-hemolytic streptococci (GBS) and gram negative enteric bacilli (e.g., E.coli), the
same organisms associated with neonatal sepsis (4). In infants and children outside of
the neonatal period, viruses are the most common cause of pneumonia (4,6,10-11) and
respiratory syncytial virus (RSV) is one of the most common causes in infancy,
especially in premature infants (9,12). Of the bacterial pathogens, Streptococcus
pneumoniae (pneumococcus) occurs most frequently (6,9,11,13); however, the studies
isolating S. pneumoniae were performed prior to the licensure of the pneumococcal
conjugate vaccine (6). Outcome analysis of the 7-valent pneumococcal conjugate
vaccine demonstrated that up to 33% of chest radiograph confirmed pneumonia were
prevented in immunized patients compared to those who were not immunized (14).
Therefore, a different bacterial pathogen may supersede S. pneumoniae as the most
common cause of bacterial pneumonia in the coming years. Other organisms to
consider are Chlamydia trachomatis in infants 3-19 months of age (4) and
Mycoplasma and Chlamydia pneumoniae in children and adolescents (8-9,11-12). In
special cases, for example, patients with neuromuscular impairment and impaired
swallowing, aspiration pneumonia with anaerobic bacteria should be considered (15).
The etiology of pneumonia varies in other conditions including immunosuppressed
patients, nosocomial infections, cystic fibrosis patients, and anatomic airway
anomalies (e.g., tracheostomies). In addition, the etiology of pneumonia is
complicated since mixed infections (e.g., viral-bacterial) can occur in 16-34% of
patients (7,11,13).
The lower respiratory tract in healthy persons is sterile (16). Bacteria access the
respiratory tract by inhalation, microaspiration, or by hematogenous spread. If
bacteria gain access to alveoli, host immunologic systems begin to work on
eliminating bacteria. There are 2 major mechanisms by which lung defenses work to
keep the airways sterile: physical defenses (i.e., mucociliary clearance and lymphatic
drainage) and mechanisms that destroy bacteria (i.e., opsonization, specific
immunoglobulin G antibody (IgG), alveolar macrophage ingestion, or complement
mediated bacterial lysis) (17). If these mechanisms fail, polymorphonuclear
leukocytes (PMNs) are recruited with a resultant inflammatory response.
Perpetuation of this inflammatory response leads to pneumonia. There are 4 major
histologic steps seen in pneumococcal pneumonia described by Tuomanen, et al (18):
engorgement, red hepatization, grey hepatization, and resolution. Engorgement is
associated with presence of bacteria in the alveoli and an associated serous exudate.
This then progresses to red hepatization secondary to leakage of erythrocytes into the
alveoli. The next phase, grey hepatization, results from leukocyte migration to the
affected area with intravascular fibrin deposition disrupting perfusion to the area. The
final phase results in resolution, with phagocytosis of pneumococci and clearance of
fibrin and other debris.
Outside of the neonatal period, pneumonia is suspected in patients with clinical signs
and symptoms suggestive of impairment of the lower respiratory tract. Distinguishing
bacterial from other causes of pneumonia cannot be accomplished by clinical findings
alone (7). Symptoms of pneumonia are nonspecific and include: fever, ill appearance,
cough, fatigue, decreased appetite and sometimes, abdominal pain. Signs of lower
respiratory tract involvement include: tachypnea (greater than 50 breaths/minute in
children less than 12 months, and greater than 40 breaths/minute for older children)
(4), cyanosis, increased work of breathing (i.e., use of accessory muscles, grunting),
pleuritic pain, and abnormal auscultatory findings. These signs do not differentiate a
viral from bacterial process. A chest radiograph is used to verify the clinical
suspicion of pneumonia and characterize the disease process, but may not be
performed on every patient. Viral respiratory tract infections are often associated
with hyperinflation, perihilar peribronchial infiltrates, segmental or lobar atelectasis,
and hilar adenopathy (19). Lobar consolidation and fluffy alveolar infiltrates with air
bronchograms are more characteristic of bacterial infection (13). However, there is
overlap between these two groups (13). Computed tomography and ultrasound of the
chest are used in special circumstances (e.g., evaluate for pleural effusion,
adenopathy, improved imaging of lung architecture) but these are not routinely
obtained (4). Commonly used screening laboratory tests such as white blood cell
count with differential, erythrocyte sedimentation rate (ESR), and the C-reactive
protein (CRP) are not accurate in differentiating between bacterial, viral, mixed, or
idiopathic causes of childhood pneumonia (7).
Determination of precise etiology of pneumonia is difficult due to the lack of sensitive
and specific tests. Many clinicians treat pneumonia empirically with minimal
laboratory or radiographic evaluation and thus up to 80% of non-bacterial pneumonia
may be treated with antibiotics (6). This approach is satisfactory when clinical risk is
deemed to be low. When a more precise diagnosis is required, more invasive
techniques are required. Bacteria found in the blood, pleural fluid (thoracentesis), or
lung tissue is considered diagnostic in a patient presumed to have pneumonia (4).
Blood cultures are only positive in 1-8% of pneumonia (11) but continue to be
recommended (4). Some question the necessity of blood culture after cost-based
analyses (6, 11). Transthoracic needle aspirates, transtracheal aspirates, and open
lung biopsy (the gold standard for diagnosis) are rarely performed due to the risk
involved for these procedures (11,20), except in severe cases or in
immunocompromised hosts (4). Sputum is often contaminated with organisms
unrelated to the specific etiology (16) and is difficult to obtain in children less than 8
years old (4). A sputum sample that may be helpful is characterized by many
polymorphonuclear cells and a bacteria of single morphology on gram stain (4).
Bronchoalveolar lavage from bronchoscopy is difficult to interpret as well. The
results are non-specific (i.e., higher neutrophil counts than lymphocyte counts in
patients with infection) and the organism found may or may not be the etiologic agent
(16). Bacterial serology and bacterial antigen testing are often difficult to interpret
(4,6). Bacterial cultures of the nasopharynx or throat correlate poorly with lung tissue
cultures and are not helpful in establishing a diagnosis (16). Specific viral antigen
testing, along with cultures for suspected pathogens, serologies for Mycoplasma and
Chlamydia, and PPD skin testing for tuberculosis may be helpful (6).
Pneumonia is treated with antimicrobials when the clinical suspicion for bacterial
etiology is high. Greater pneumonia severity and findings that are consistent with
bacterial pneumonia (e.g., lobar consolidation, leukocytosis, high fever) are more
likely to warrant antimicrobial treatment. Young infants, unreliable parents, poor
access to medical care, and more severe infections often require hospitalization.
Treatment of pneumonia is often empirically based and thus, information on antibiotic
resistance patterns and mechanisms of resistance is important to determine the most
appropriate treatment. For S. pneumoniae, the most common mechanism of
resistance to penicillins is alteration of penicillin-binding sites that can be overcome
with higher doses of the drug (6). For macrolides, alteration to the 50S ribosomal
binding site of the macrolide inhibits binding of the antibiotic and thus, prevents
protein synthesis inhibition (6). In addition, there is also an increase in efflux pumps
for macrolides and this property can be overcome by using macrolides that achieve
high tissue concentrations at the site of infection (e.g. azithromycin) (6). Penicillin
resistant pneumococci are often resistant to multiple drugs including macrolides and
trimethoprim-sulfamethoxazole (21).
Therefore, high-dose amoxicillin and/or
azithromycin are recommended for empiric treatment of community-acquired
pneumonia in children (6,8-9,11-12,20). Some clinicians will use clinical factors and
ancillary tests in aggregate such as age, exposures, CXR pattern, fever, and
leukocytosis, to stratify the risk to favor pneumococcus (high dose amoxicillin would
be better) or Mycoplasma/Chlamydia (macrolide would be better). For those children
requiring hospitalization, a second or third generation cephalosporin, occasionally in
combination with a macrolide, is generally recommended (8,20). Most treatment
regimens are continued for a total of 7-14 days although this is based on little
evidence (4).
Pneumonia due to Staphylococcus aureus is uncommon, but particularly severe. S.
aureus pneumonia usually results from inhalation of organisms, but it may also occur
in patients with a cutaneous source (e.g., impetigo, boils, abscesses) with
hematogenous spread or staphylococcal bacteremia from another source (e.g.
osteomyelitis, central line infection). If S. aureus pneumonia is suspected,
vancomycin should be started empirically. Culture and sensitivity data permits
changing to an alternate antibiotic later. Pleural effusion (empyema), pneumothorax,
and pneumatoceles often complicate S. aureus pneumonia.
Pleural effusions can be classified in several ways. They can be a transudate or an
exudate based on their protein content. A subpulmonic effusion versus an empyema
is more clinically relevant. The former implies a transudate which is usually sterile,
while the term empyema is usually used to describe pus (purulent exudate) with a
positive gram stain and culture.
The overall outcome in children with pneumonia is excellent. The majority of
children will recover without complications (11). Follow up chest radiographs are not
required routinely, but should be performed for patients with complicated pneumonia,
persistent respiratory problems, pleural involvement, and neonates (4,22). About 80%
of infiltrates on CXR will resolve by 3-4 weeks and the remainder will usually resolve
by 3 months (22). Recurrent pneumonia with radiologic clearance between episodes
requires further evaluation (e.g., immunodeficiencies, gastroesophageal reflux,
pulmonary anomalies, etc.) (4).
Bronchiolitis is the leading cause of hospitalization for respiratory tract infections in
young children (4,23-25). Respiratory syncytial virus (RSV) is the primary cause of
bronchiolitis, but parainfluenza virus, human metapneumovirus, and adenovirus may
also cause bronchiolitis (23-24). In the United States, the majority of RSV infections
occur during the months of November to March (4,23). RSV infections account for a
significant amount of morbidity and health care expense in the young age group (24).
RSV is transmitted by direct contact with large droplets or fomites. Transmission can
be limited by good handwashing (23). RSV bronchiolitis results from the spread of
RSV to the lower respiratory tract after an incubation period 2-8 days where the virus
undergoes replication in the nasopharynx (23). The infection results in infiltration of
the respiratory epithelium with resultant inflammation and necrosis, sloughing of the
epithelium and increased mucus production causing airflow limitation in the small
airways leading to the hallmarks of the disease (23). Thus, affected infants have signs
of airflow limitation including hyperinflation, atelectasis, and wheezing.
The diagnosis is often made on clinical grounds during the RSV season. Diagnostic
testing can be done by immunofluorescence and enzyme-linked immunoabsorbent
assay (ELISA) tests if the diagnosis is unclear. Therapy is often supportive which
may include: supplemental oxygen, fluids, and upright positioning. Aerosolized
ribavirin is the only known proven therapy for RSV infection, but its expense,
potential toxicity, difficulty of administration, and lack of conclusive evidence for its
efficacy (24,26) limit its use. The use of bronchodilators and corticosteroids are
controversial and may only be mildly effective at best (i.e., not been proven to be
highly efficacious) (24-26). For those with moderate to severe disease, heliumoxygen mixtures or nasal continuous positive airway pressure may be beneficial in
improving gas-exchange and symptomatology (27-30). Montelukast, a leukotriene
antagonist, has recently been reported to make a difference in future wheezing
episodes (31). Prophylaxis with palivizumab (RSV monoclonal antibody) or RSVIVIG is given to select pediatric populations recommended by the American
Academy of Pediatrics during RSV season to reduce RSV infection risk (32).
Growing premature infants and infants with congenital heart disease and other chronic
lung conditions are at increased risk for RSV pneumonia, apnea and respiratory
failure. Healthy term infants with RSV usually develop mild bronchiolitis. Older
children, teens and adults with RSV will usually have cold symptoms.
Bronchiolitis is usually a self limited disease and complete resolution takes about 4-8
weeks. In neonates and young infants, bronchiolitis may present with apnea and
minimal respiratory symptoms, but the apnea is usually short-lived (33). Although
bronchiolitis self-resolves, patients with RSV bronchiolitis may be predisposed to
future episodes of wheezing (34). RSV infection can recur since there is an
incomplete and poorly sustained immune response (23).
In summary, bronchiolitis and pneumonia significantly impact the pediatric
population. Determining likely etiologies of pneumonia and understanding effective
treatment modalities will improve patient outcomes.
Questions
1. Which of the following is the most common cause of pneumonia outside of the
neonatal period?
a. S. pneumoniae
b. Mycoplasma
c. Viruses
d. Chlamydia
2. S. pneumonia resistance to penicillins is due to:
a. Production of beta-lactamase
b. Alteration of penicillin binding proteins
c. Increased efflux pumps
d. Low tissue bioavailability
3. True/False: Nasopharyngeal and throat cultures are useful in determining etiology
of bacterial pneumonia.
4. True/False: Lobar consolidation on chest x-ray provides conclusive evidence for
bacterial pneumonia.
5. Which factor does not appear to affect the etiology of pneumonia?
a. Age
b. Vaccination status
c. Current antibiotic use
d. Birth rank
6. The most common cause of bronchiolitis is:
a. Respiratory syncytial virus
b. Human Metapneumovirus
c. Parainfluenza
d. Adenovirus
7. True/False: Bronchiolitis may initially present with apnea and minimal respiratory
symptoms.
8. Treatment of bronchiolitis should include all of the following except:
a. Supplemental oxygen for infants with hypoxia.
b. Intravenous fluids and close monitoring of nutritional status.
c. Good handwashing.
d. Antibiotics.
9. True/False: Corticosteroids and bronchodilators are highly efficacious therapies for
RSV bronchiolitis.
Answers to questions
1.c. Overall, viruses cause the majority of pneumonias in children; however,
the incidence of viral pneumonia decreases with age, becoming less common in older
children and adolescents.
2.b
3.False
4.False. Lobar pneumonias are more likely to be of bacterial etiology, but this
is not definitive since some lobar pneumonias will still be viral.
5.d
6.a
7.True
8.d
9.False
References
1. Graham NMH. The Epidemiology of Acute Respiratory Infection in
Children and Adults: A Global Perspective. Epidemiol Rev 1990;12:149-178.
2. National Center for Health Statistics. National Vital Statistics report
(www.cdc.gov) 2001;49(1):14.
3. Dixon RE. Economic Costs of Respiratory Tract Infections in the United
States. Am J Med 1985;78(6B): 45-51.
4. Miller MA, Ben-Ami T, Daum RS. Chapter 39-Bacterial Pneumonia in
Neonates and Older Children. In: Taussig LM, Landau LI (eds). Pediatric
Respiratory Medicine, first edition. 1999, St. Louis: Mosby, pp. 595-664.
5. Dowell SF, Kupronis BA, Zell ER, Shay DK. Mortality From Pneumonia
in Children in the United States, 1939 Through 1996.
N Engl J Med
2000;342(19):1399-1407.
6. Bradley JS. Management of Community-Acquired Pediatric Pneumonia in
an Era of Increasing Antibiotic Resistance and Conjugate Vaccines. Pediatr Infect
Dis J 2002;21(6):592-598.
7. Nohynek H, Valkeila E, Leinonen M, Eskola J. Erythrocyte Sedimentation
Rate, White Blood Cell Count and Serum C-reactive Protein in Assessing Etiologic
Diagnosis of Acute Lower Respiratory Infections in Children. Pediatr Infect Dis J
1995;14(6): 484-490.
8. Nelson J. Community-Acquired Pneumonia in Children: Guidelines for
Treatment. Pediatr Infect Dis J 2000;19(3):251-253.
9. Heiskanen-Kosma T, Korppi MN, Jokinen C, et al. Etiology of Childhood
Pneumonia: Serologic Results of a Prospective, Population-Based Study. Pediatr
Infect Dis J 1998;17(11):986-991.
10. McIntosh K. Community-Acquired Pneumonia in Children. N Engl J
Med 2002;346(6);429-437.
11. Ruuskanen O, Mertsolon J. Childhood Community-Acquired Pneumonia.
Sem Resp Infect 1999;14(2):163-172.
12. McCraken, GH Jr. Etiology and Treatment of Pneumonia. Pediatr Infect
Dis J 2000;19(4):373-377.
13. Korppi M, Kiekara O, Heiskanen-Kosma T, Soimakallio S. Comparison
of Radiological Findings and Microbial Aetiology of Childhood Pneumonia. Acta
Paediatr 1993;82: 360-363.
14. Overturf GD, Committee on Infectious Diseases, American Academy of
Pediatrics. Technical Report: Prevention of Pneumococcal Infections, Including the
Use of Pneumococcal Conjugate and Polysaccharide Vaccines and Antibiotic
Prophylaxis. Pediatrics 2000;106(2):367-376.
15. Brook I, Finegold SM. Bacteriology of Aspiration Pneumonia in
Children. Pediatrics 1980;65(6):1115-1120.
16. Laurenzi GA, Potter RT, Kass EH. Bacteriologic Flora of the Lower
Respiratory Tract. N Engl J Med 1961;265:1271-1278.
17. Green GM, Kass EH. The Role of the Alveolar Macrophage in the
Clearance of Bacteria from the Lung. J Exp Med 1964;119:167-175.
18. Tuomanen EI, Austrian R, Masure HR. Pathogenesis of Pneumococcal
Infection. N Engl J Med 1995(19);332:1280-1284.
19. Wildin SR, Chonmaitree T, Swischuk LE. Roentgenographic Features of
Common Pediatric Viral Respiratory Tract Infections. Am J Dis Child 1988;142:4346.
20. Hart CA, Duerden BI. Respiratory Infections. J Med Microbiol
2002;51:903-914.
21. Whitney CG, Farley MM, Hadler J, et al. Increasing Prevalence of
Multidrug-Resistant Streptococcus Pneumoniae in the United States. N Engl J Med
2000;343(26):1917-1924.
22. Grossman LK, Wald ER, Nair P, Papiez J. Roentgenographic Follow-Up
of Acute Pneumonia in Children. Pediatrics 1979(1);63:30-31.
23. Hall CB. Respiratory Syncytial Virus and Parainfluenza virus. N Engl J
Med 2001;344(25):1917-1928.
24. Greenough A. Respiratory Syncytial Virus Infection: Clinical Features,
Management, and Prophylaxis. Curr Opin in Pulm Med 2002;8:214-217.
25. Jartti T, van den Hoogen B, Garofalo RP, et al. Metapneumovirus and
Acute Wheezing in Children. Lancet 2002;360:1393-1394.
26. Patel H, Platt RW, Pekeles GS, Ducharme FM. A Randomized
Controlled Trial of the Effectiveness of Nebulized Therapy with Epinephrine
Compared with Albuterol and Saline in Infants Hospitalized for Acute Viral
Bronchiolitis. J Pediatr 2002(6);141:818-824.
27. Hollman G, Shen G, Zeng L, et al. Helium-Oxygen Improves Clinical
Asthma Scores in Children with Acute Bronchiolitis.
Crit Care Med
1998;26(10):1731-1736.
28. Martinon-Torres F, Rodriguez-Nunez A, Martinon-Sanchez JM. Heliox
Therapy in Infants with Acute Bronchiolitis. Pediatrics 2002(1);109:68-73.
29. Beasley JM, Jones SEF. Continuous Positive Airway Pressure in
Bronchiolitis. British Medical Journal 1981;283:1506-1508.
30. Soong W, Hwang B, Tang R. Continuous Positive Airway Pressure by
Nasal Prongs in Bronchiolitis. Pediatr Pulmonol 1993;16:163-166.
31. Bisgaard H for the Study Group on Montelukast and Respiratory
Syncytial Virus. A Randomized Trial of Montelukast in Respiratory Syncytial Virus
Postbronchiolitis. Am J Respir Crit Care Med 2003;167:379-383.
32. Halsey NA, Abramson JS, Chesney PJ, et al. American Academy of
Pediatrics: Prevention of Respiratory Syncytial Virus Infections: Indications for the
Use of Palivizumab and Update on the Use of RSV-IGIV.
Pediatrics
1998;102(5):1211-1216.
33. Bruhn FW, Mokrohisky ST, McIntosh K. Apnea Associated with
Respiratory Syncytial Virus Infection in Young Infants. J Pediatr 1977;90:382-386.
34. Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory
Syncytial Virus Bronchiolitis in Infancy is an Important Risk Factor for Asthma and
Allergy at Age 7. Am J Respir Crit Care Med 2000;161:1501-1507.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
Asthma:
A three year old comes in with a complaint of coughing for 2 weeks. Coughing is
present every night. He has also had a mild fever, but his temperature has not been
measured at home. His parents have been using a decongestant/antihistamine syrup
and albuterol syrup which were left over from a sibling. Initially the cough improved
but it worsened over the next 2 days. He is noted to have morning sneezing and nasal
congestion. There are colds going around the pre-school. He has had similar
episodes in the past, but this episode is worse. He has no known allergies to foods or
medications.
His past history is notable for eczema and dry skin since infancy. He is otherwise
healthy and he is fully immunized. His family history is notable for a brother who has
asthma. In his home environment, there are no smokers or pets.
Exam: VS T 38.1, P 100, RR 24, BP 85/65, oxygen saturation 99% in room air. He
is alert and cooperative in minimal distress if any. His eyes are clear, nasal mucosa is
boggy with clear discharge, and his pharynx has moderate lymphoid hypertrophy. He
has multiple small lymph nodes palpable in his upper neck. His chest has an
increased AP diameter and it is tympanitic (hyperresonant) to percussion. Rhonchi
and occasional wheezes are heard on auscultation, but there are no retractions. Heart
is in a regular rhythm and no murmurs are heard. His skin is dry, but not flaky,
inflamed or thickened.
He is initially felt to have moderately persistent asthma and possible asthmatic
bronchitis.
He is initially treated with nebulized albuterol and nebulized
corticosteroids for bronchospasm and bronchial inflammation. He is also treated with
an antihistamine at night to reduce his morning allergy symptoms. In follow-up, his
cough does not improve and he is still having fever (T 38.2C, 101.0F). A chest X-ray
is obtained, but no radiographic evidence of pneumonia is present. His cough
persists, but only with exercise and drinking cold juice.
His chest now sounds clear in the office. After one week of no night cough, his
nebulized albuterol+corticosteroid is reduced to 2 times a day. His exercise induced
cough gradually resolves. His nebulized corticosteroid is replaced with nebulized
cromolyn twice a day and oral montelukast (a leukotriene inhibitor) is added. He
enrolls in a soccer league and plays with minimal coughing. His routine nebulized
albuterol+cromolyn is stopped and is used only pre-exercise to prevent exercise
induced bronchospasm. No cough is observed at night or with exercise. He is
continued on nightly antihistamines, pre-exercise albuterol+cromolyn nebs, and once
daily montelukast. He is given an asthma treatment plan which gives his parents clear
instructions on which medications to start based on his symptoms and severity.
Asthma is by far, the most frequent respiratory diagnosis for children admitted to
hospitals. It causes 5000 deaths annually in the United States despite the availability
of excellent medications. Historically, asthma was characterized as a psychological
illness, a surgical illness treated by removal of the carotid body, an environmental
illness aggravated by air pollution, and an allergic illness or infectious illness. An
allergy role in asthma, was legitimized by the discovery of IgE in 1965. Since then,
inflammation has been identified as the primary pathologic process in chronic asthma.
Because of the variety of asthma triggers, such as exercise, exposure to smoke,
weather changes, and allergies, asthma is now considered to be a syndrome consisting
of bronchospasm, airway hyperirritability, and inflammation. The popular term
ROAD (reversible obstructed airways disease) or RAD (reversible airway disease) is
not entirely accurate since this is only part of the disease process and reversibility may
not always be evident. This is because obstruction of the airways may be secondary
to mucous plugging or inflammatory changes decreasing the caliber of the airways, in
which case, beta-2 bronchodilators are ineffective.
Currently the NIH Guidelines (1) have served as a standard for diagnosing and
treating asthma. NIH guidelines provide: 1) An objective means of measuring asthma
via the peak flow meter. 2) A way of objectively categorizing severity classes of
patients based on symptoms and/or peak flow measurements. 3) A comprehensive
pharmacologic plan primarily designed to treat inflammation, inclusive of provisions
for acute and maintenance care, for each severity level. 4) For the identification and
removal of (or control of exposure to) known triggers. 5) The direction for forming a
partnership with the physician who uses education as a primary basis of this
relationship.
The realization that IgE existed and could be found in allergic individuals propelled
the field of allergy and understanding of asthma into a renaissance of elucidating the
actual pathophysiology of allergic diseases. Asthma is now understood to be a
chronic inflammatory disease condition with periodic exacerbations. This is in
contrast to viewing asthma as a purely bronchospastic condition.
An acute asthma exacerbation is a biphasic process. Understanding the inflammatory
process of asthma came about when it was observed that 4 to 8 hours following
allergen exposure, wheezing would occur that was not responsive (or less responsive)
to beta agonists but it was ablated by cromolyn and corticosteroids. However, beta
agonists could easily neutralize the immediate reaction, occurring within minutes of
the allergen exposure. This created a picture of a biphasic reaction to allergen (or
infection) induced wheezing. The first phase was described as the immediate
(bronchospastic) phase and the second phase as the late phase inflammatory response.
In the early phase of allergic inflammation, preformed mediators such as histamine
and rapidly formed mediators such as leukotrienes are released and cause
bronchospasm. Other mediators signal the late phase inflammatory cells. These cells
(e.g., eosinophils) recruit other cells such as epithelial cells to participate in the
resultant inflammatory damage of the airways and subepithelial structures. These
events eventually result in extensive restructuring of the normal histology of the
airways. This damage is not restored by beta-2 bronchodilators. An important
immunologic occurrence is the activation of the Th2 helper cell, which is pivotal in
the progression of the allergic immunologic process. The other helper designated Th1
cell does not enhance the allergic inflammatory process.
Asthma, whatever the severity, is a chronic inflammatory disorder of the airways.
The characteristic features of asthmatic inflammation are: mast cell activation,
inflammatory cell infiltration, eosinophils, macrophages, neutrophils (particularly in
sudden-onset, fatal exacerbations), lymphocytes (TH2-like cells), edema, denudation
and disruption of the bronchiolar epithelium, collagen deposition beneath the
basement membrane (this is an irreversible process), goblet cell hyperplasia, mucous
hypersecretion, and smooth muscle thickening. The primary clinical components of
asthma include: bronchospasm, inflammation, airway hyper-reactivity, increased
mucous production, and end expiratory hyperinflation ("air trapping").
There are many presentations of asthma. Asthma is present 24 hours a day, 7 days a
week. It may not be in an easily identified form (i.e., there may be no obvious
symptoms present). The most recognizable form is the acute episode in which the
patient presents with acute shortness of breath. Depending on the underlying degree
of inflammatory damage of the airways, the episode may have been festering with
persistent cough and occasional bouts of shortness of breath for weeks. Failure to
attend to these soft signs of "asthma in transition" may lead to an acute case of status
asthmaticus. Hence, paying attention to signs of "silent asthma" (asthma not in an
acute phase), can prevent costly and life threatening consequences. Asthma may
appear solely as an event associated with work or exercise. Most asthma in childhood
occurs as a result of encounters with respiratory viruses. If the asthmatic is already
unstable because of a poor maintenance regimen of the existing chronic asthma, the
acute phase will begin simultaneously with the first signs of a "cold". If the asthma is
managed well, then the cough and wheezing may occur several days after cold
symptoms. Hence, early recognition of "asthma in transition" is a major point of
cooperation involving the physician and patient. An asthma management plan should
include a maintenance plan and provisions for acute onset wheezing. Asthma in its
most manageable state, is outpatient asthma, as opposed to hospital status
asthmaticus.
For most medical professionals, the first and everlasting impression of asthma is in
hospital status asthmaticus. By far, the more common situation is asthma outside the
hospital, in its non-acute form. Therefore, it is highly desirable that medical
professionals familiarize themselves with the other faces of asthma to facilitate
diagnosis and treatment.
The type of medication used to treat asthma reflects the mechanism of airway
obstruction: bronchospasm versus inflammation. This is an extremely simplified
version of what really goes on and new pieces of the intricate mechanism are being
uncovered. However from a pragmatic standpoint, the logic for appropriate use of
individual medications for asthma can be understood by recalling the biphasic
reaction.
Based on this brief description of the mechanism of asthma, it is now possible to
create an asthma treatment program. Genetics aside, elimination of triggers and
aggravators of asthma such as allergens, cigarette smoke, and environmental and
industrial pollutants, can prevent acute exacerbations of asthma and serve as the first
line of defense. Conditions such as weather changes and respiratory infections fall
outside of the readily controllable factors.
Approach to Asthma
1. Diagnose asthma and classify severity. Identify aggravating and triggering
conditions.
2. Prepare an initial treatment plan to stabilize the acute condition. Instruct
patient and parents on signs and symptoms which help to monitor the effectiveness of
treatment. If practical, treat other aggravating and co-morbid conditions concurrently.
3. When asthma is stable, proceed to a maintenance plan to allow healing of
the damaged airways. This may take weeks to months. Prepare an asthma action plan
for up-regulation of medications for unexpected exacerbations.
4. When there are no signs of breakthrough cough or wheezing, indicating
that the airway hyper-reactivity has subsided and is controlled, switch to a long term
maintenance plan. This might be PRN use of bronchodilators, or pre-exercise use of
preventive medications, or pulsing of medications for cold symptoms in short bursts.
5. Monitor asthma with periodic evaluations and reminder messages of
avoidance and check on patients' inhalation technique of medication administration.
Inflammation in asthma contributes to: airway hyperresponsiveness, airflow
limitation, respiratory symptoms, coughing, wheezing, shortness of breath, rapid
breathing, chest tightness, persistent symptoms, and pathologic damage, even when
symptoms are not present. It is often thought that periodic control of acute symptoms
is sufficient, but this is suboptimal. Utilization of chronic anti-inflammatory agents
result in better long term outcomes for all but the mildest asthmatics.
Co-morbid conditions such as allergic rhinitis, sinusitis, eczema, and
gastroesophageal reflux have profound influence on asthma. Their presence makes
asthma extremely difficult to control. The main goal is to keep the patient functional
and free of side effects from medications. With this approach, asthmatics have been
able to participate in a normal life style.
Asthma is more than an acute process. A large part of treating asthma successfully is
to be able to recognize asthma in its early stages and to formulate an appropriate
treatment plan before the asthma advances to a critical stage. It is simple to diagnose
asthma when the patient is wheezing, displaying intercostal retractions and turning
pale or blue. Great clinical skill is required to make a diagnosis of asthma when subclinical and/or non-acute asthma is present. A careful detailed history and physical
exam are crucial to this end. Asthma is not the acute episode of wheezing as
popularly described in lay journals and magazines, but a chronic condition of the
airways of the lungs which exhibits recurrent bronchospasm. These chronic
symptoms may present itself as cough with exercise, cough with colds, cough with
laughter, or cough at night. A peak flow meter can consistently record airflow
readings compared against normal values for sex and age.
Signs of "silent asthma" (when no wheezing is heard) include: persistent cough at
night, cough with exercise, cough with laughter, cough when consuming cold foods or
drinks, prolonged cough following or accompanying a cold, feeling of "tight chest" or
difficulty breathing.
The peak flow measurement and FEV1 (forced expiratory volume over one second)
are effort dependent measures. Full pulmonary function testing is desirable; however,
the equipment is expensive compared to an inexpensive peak flow meter. The
ultimate objective measurement for asthma is by body plethysmography (body box),
which can measure the end expiratory residual lung volume as well as resistance to
airflow. For those patients unable to perform peak flow measurements, clinical
history is all you may have to base your conclusions. This includes a major group of
younger asthmatics from infancy to 4 or 5 years old. Many children in this age group
are unable to reliably perform peak flow measurements.
Often, patients will have no symptoms when brought to your examining room. The
identification of the role of allergic diseases in asthma relies heavily on patient
history. Physicians trained to respond to record what they feel, see, and hear may
have a problem forming conclusions based on history alone. Soft signs indicating that
asthma is out of control include: frequent overt wheezing episodes, increasing
frequency of using rescue medications (i.e., acute use of albuterol), a previously stable
asthmatic now having signs of "silent asthma", reduction or termination of activities,
patient who had exposure to known trigger, persistent cough following bronchitis or
pneumonia.
The National Institutes of Health (NIH) guidelines, list as one of several key
objectives, forming a partnership with the patient to facilitate treatment of asthma.
Good communication and availability to answer questions and concerns are basic to
the partnership. Part of your efforts as the treating physician should be focused on
getting the patient to respond in a logical manner to cope with changes in his/her
clinical state. This is based on the patient understanding the principles of: triggers
and aggravators, bronchodilation, inflammation, airway hyper-reactivity and healing.
Patients must also understand mucous mobilization and signs and symptoms of
asthma out of control which may lead to an acute asthma attack. For example, should
the peak flow fall or cough increase, the patient is instructed to upgrade their
medications according to a prearranged plan. As the acuteness of the situation
resolves, the patient is advised to downgrade their medications back to their
maintenance program. Should there be an unanticipated episode of wheezing,
immediate activation of the action plan and consultation with the physician for
additional treatment schemes is the next step. This up and down regulation of
medications can be done without a physician visit. Phone calls, informing the
physician's office of these maneuvers, are all that is normally required. Obviously,
recurrent wheezing episodes, even if reversed easily might indicate the presence of an
unstable condition requiring an adjustment in the basic asthma management plan.
Hence, the physician should be apprised of these changing conditions regularly. All
asthma management plans should have common goals.
Asthma management plans depend on the severity of the asthmatic. Higher severity
levels warrant greater use of corticosteroids and prophylactic medications such as
leukotriene inhibitors and inhaled corticosteroids. The NIH guidelines categorizes
severity levels into "steps" as follows:
Step 1 (mild intermittent): Day symptoms two days per week or less and night
symptoms two nights per month or less. Chronic peak flow is 80% of expected or
higher.
Step 2 (mild persistent): Day symptoms greater than two times per week, but
less than once per day or night symptoms greater than nights per month. Chronic
peak flow is still 80% of expected or higher.
Step 3 (moderate persistent): Day symptoms occur daily or night symptoms
occur more than once per week. Chronic peak flow is 60% to 80% of expected value.
Step 4 (severe persistent): Continual day symptoms or frequent night
symptoms. Chronic peak flow is less than or equal to 60% of expected value.
The use of peak flow in the above classification is not required in children 5 years and
under. Peak flow data is useful but not required for classification in older age groups,
but most children in this age range are capable of performing peak flows.
The major goal is to allow the child to express and achieve his or her maximum
natural potential by not allowing the asthma to control him or her. This is a good way
to view the end point in asthma management. Along the way, it is crucial to cradle
the impressionable self image so that the child does not have a negative view of
himself or herself. The very impressionable years are from about 3 to 10 years of age,
when children form their life-long mental image of themselves. Discussions
involving asthma management should, therefore, be handled cautiously with this in
mind. Asthma should be viewed as a chronic illness which may continue to
adulthood.
Bronchodilators
In 1896 Solis-Cohen published, "The use of adrenal substances in the treatment of
asthma" (adrenalin or epinephrine is a fast and potent bronchodilator). Epinephrine
(most commonly administered subcutaneously, but it could be inhaled as well) was
the first line of treatment for acute asthma from the 1950s through the 1970s and early
1980s.
In 1924 ephedrine was isolated from Ma Huang (a Chinese root extract). For the next
forty years, ephedrine would be the mainstay for asthma treatment in the USA.
Ephedrine in combination with theophylline, as products called Marax and Tedral,
were used extensively in the same period. Interestingly, the ancient Chinese boiled
the ephedra root in strong tea for their concoction to treat asthma. The tea contained
theobromine, a methylxanthine. Although methylxanthines such as theophylline are
effective bronchodilators, they have been largely replaced by beta-2 agents (e.g.,
albuterol) which have a faster onset and less toxicity. Adding theophylline does not
appear to acutely benefit most patients who are receiving high therapeutic doses of
albuterol. Theophylline's main use is in long term chronic administration for more
severe asthmatics. This change in therapeutic approach from methylxanthines to beta2 agents did not further our understanding of the true pathophysiology of asthma, as
bronchodilation was the only target of treatment.
Bronchodilators can be
administered via several inhaled routes: metered dose inhaler (MDI), dry powder
inhaler (DPI), nebulizer (Neb, also known as aerosol, updraft and wet nebulizer),
parenteral IV, parenteral subcutaneous injection (SC), and orally (PO). In general,
inhaled medications have a faster onset, greater potency and less side effects.
Bronchodilators Used in Asthma
A. Beta-2 Agonists:
albuterol (Ventolin, Proventil, also called salbutamol outside the USA) - MDI,
Neb, PO
L-albuterol (Xopenex - active isomer only) - Neb
terbutaline - MDI, Neb, PO, SC
formoterol (Foradil - very long acting) - DPI
salmeterol (Serevent - used for maintenance therapy) - DPI, MDI
epinephrine (alpha and beta) - MDI, Neb, SC
B. Anticholinergics
ipratropium bromide (Atrovent) -MDI, Neb
oxitropium bromide (Oxivent) - MDI
atropine -Neb
C. Methylxanthines
aminophylline - PO, IV
theophylline - PO, IV
oxtriphylline - PO
Other drugs with bronchodilator effects include ketamine, calcium channel blockers
(e.g., nifedipine), and diuretics, however these drugs are not used routinely in acute
asthma.
Anti-Inflammatory Drugs
Based on the biphasic mechanism, an anti-inflammatory drug (i.e., corticosteroids) is
necessary for the complete treatment of asthma. Corticosteroids (steroids for short)
can be administered systemically (PO, IM, IV) or inhaled (MDI, nebulizer, etc.). For
asthma of a chronic nature, such as allergic asthma to house dust, a daily regimen of a
long acting bronchodilator coupled with a steroid by inhalation would be effective.
Steroids take hours to become engaged in its active phase. Their action does not take
place directly on the inflammatory tissue but by modulating DNA production of proinflammatory cytokines. Their effects are very broad and nonspecific. Steroids affect
virtually every phase of the inflammatory process. They have an array of impressive
and undesirable side-effects, which cause hesitation in their use by physicians as well
as patients. As in the use of any medication or therapeutic agent, the employment of
steroids is subject to weighing the desired effects against the undesirable effects
(benefit vs. risk). If the positive effects of using steroids have an overwhelming
advantage over not using the drug, then it is justified to be used on a regular basis.
This especially applies to children where growth suppression (in the order of 0.5 to
1.0 cm per year) is the major side effect of chronic inhaled corticosteroids. Catch-up
growth occurs in most instances, if the child's condition improves to the point at
which inhaled corticosteroids are no longer needed. Many patients require more
medications during the fall/winter/spring, and fewer medications during the summer.
Occasional bursts of systemic corticosteroids have no significant long term side
effects, but chronic or long term use of systemic steroids have major side effects
(refer to the chapter on corticosteroids).
Corticosteroids used in Asthma
beclomethasone (Beclovent, Vanceril) - inhaled
triamcinolone (Azmacort) - inhaled, IM
budesonide (Pulmicort) - inhaled
fluticasone (Flovent) - inhaled
flunisolide (AeroBid) - inhaled
mometasone (Asmanex) - inhaled
prednisone - PO
prednisolone (Pediapred, Prelone, Orapred) - PO
methylprednisolone (Medrol, Solumedrol) - PO, IV
dexamethasone (Decadron) - PO, IV
In addition, one might consider adding a leukotriene inhibitor, also called
leukotriene receptor antagonists (LTRA).
These leukotriene inhibitors were
developed to counteract the all important late phase inflammatory reaction caused by
SRS-A (slow reacting substance of anaphylaxis), a compound which was eventually
identified as leukotrienes. Their side effects are minimal. These are all given orally.
Leukotriene receptor antagonists (LTRA)
montelukast* (Singulair)
zafirlukast* (Accolate)
pranlukast
zileuton (Zyflo)
*(Some sources spell the suffix as "leukast" instead of "lukast". Roche and Astra
Zeneca spell it as "lukast".)
Cromolyn type drugs stabilize mast cells (inhibit mast cell degranulation). They have
less potent anti-inflammatory properties, but they have minimal side effects.
Cromolyn (Intal) is available via nebulizer and MDI. Nedocromil (Tilade) is
available via MDI.
Goals of Asthma Treatment
1. Prevent chronic and troublesome symptoms (e.g., cough or breathlessness
in the night, in the early morning, or after exertion).
2. Maintain (near) "normal" pulmonary function.
3. Maintain normal activity levels (including exercise and other physical
activity).
4. Prevent recurrent exacerbations of asthma and minimize the need for
emergency department visits or hospitalizations.
5. Provide optimal pharmacotherapy with minimal or no adverse effects.
6. Meet patients' and families' expectations of and satisfaction with asthma
care.
Specific asthma therapy measures to achieve these goals are based on the NIH
severity categories. Step 1 (mild intermittent) requires no daily medications. ALL of
the other categories (i.e., any category with the word "persistent"), requires a chronic
controller anti-inflammatory medication.
Step 2 (mild persistent) recommends a low dose inhaled corticosteroid. Alternatively,
a cromolyn medication or a leukotriene receptor antagonist may be used.
Theophylline is another option, but only in children older than 5 years.
Step 3 (moderate persistent) recommends a low dose inhaled corticosteroid plus a
long acting beta-2 agonist (salmeterol or formoterol). Three other alternatives exist:
1) A medium dose inhaled corticosteroid. 2) A low dose inhaled corticosteroid plus
an LTRA. 3) A low dose inhaled corticosteroid plus theophylline.
Step 4 (severe persistent) recommends a high dose inhaled corticosteroid, plus a long
acting beta-2 agonist.
In addition to the above chronic (long-term) recommendations, acute exacerbations
are treated with quick relief (or rescue) medications, which is most commonly prn
albuterol and optional short bursts of systemic corticosteroids. Albuterol can be
given: 1) Orally at 0.1 mg/kg per dose every 6 to 8 hours. 2) Via nebulizer 2.5 mg
unit dose every 4-6 hours. 3) Via metered dose inhaler (MDI) 2-4 puffs every 4-6
hours (however, most studies suggest that 5 to 10 puffs is more equivalent to the 2.5
mg nebulizer treatment).
Systemic corticosteroids are commonly administered as: 1) Oral prednisolone at 2
mg/kg/day given once daily or divided BID. 2) IV methylprednisolone 2 mg/kg, then
1 mg/kg every 6 hours. Systemic corticosteroids are usually given for 4 to 5 days and
then discontinued if the patient improves. Systemic corticosteroids administered for
longer than 7 days require a gradual taper of the medication. If the patient is on
inhaled corticosteroids, these should be resumed once systemic corticosteroids are
stopped or tapered. Some physicians continue inhaled corticosteroids during systemic
corticosteroid bursts to avoid the confusion caused by modifying their chronic
medications.
All patients should have a written asthma management plan that describes their
chronic medications and a plan for the initiation of a rescue plan based on their
symptoms and peak flow (if age >5 years). More detailed plans can include
recommendations to step up or step down their chronic medications as their chronic
symptoms worsen or improve.
If dyspnea still persists, despite rescue medications, then the asthma management plan
should refer the patient to a source of immediate medical care (doctor's office during
office hours, or emergency room after hours). Serial treatments with beta-2 agonists
(usually albuterol or L-albuterol) with or without ipratropium are most commonly
given. Inhaled beta-2 agonists can be given continuously for severely ill patients, or
serially based on severity. Systemic corticosteroids can be started. Parenteral
corticosteroids do not have an onset time advantage over oral corticosteroids;
however, very ill children have a higher likelihood of vomiting oral prednisolone.
Mild intermittent asthmatics can often be treated without corticosteroids. The
decision to start systemic corticosteroids is based on their response to beta-2 agonists
and their previous history which indicates their severity level. Those who do not
respond well to beta-2 agonists should be started on systemic corticosteroids because,
poor response indicates the presence of significant bronchial inflammation Those
who have required systemic corticosteroids in the past or who have other markers of
more severe asthma should also be started on systemic corticosteroids.
Characteristics of good asthma control in children include: no coughing, no shortness
of breath or rapid breathing, no wheezing or chest tightness, no waking up at night
because of asthma symptoms, normal activities including play, sports, and exercise,
no episodes of asthma that require a doctor visit, emergency room visit, or urgent
care, no absences from school or activities, no missed time from work for the parent
or caregiver, normal or near normal lung function, and a healthy self image (i.e.,
"nothing can stop me attitude").
Unfortunately, the death rate from asthma is not yielding to the introduction of many
excellent and powerful treatments. This condition remains a challenge to the medical
care team at all levels from physicians, nurses, emergency technicians, and respiratory
therapists to psychiatrists and social workers. Family, school personnel, coaches, club
leaders, and after hours activity supervisors, are all involved in delivering care to the
asthmatic.
Risk factors for death from asthma include:
Past history of sudden severe exacerbations.
Prior intubation for asthma.
Prior admission to intensive care unit for asthma.
Greater than 2 hospitalizations for asthma in the past 12 months.
Greater than 3 emergency room visits for asthma in past 12 months.
Hospitalization or emergency care visit for asthma in the past month.
Use of more than 1 canister per month of inhaled short-acting beta 2 agonist.
Current chronic use of oral corticosteroids.
Difficulty perceiving airflow obstruction or its severity.
Low socioeconomic status and urban residence.
Illicit (illegal) drug use.
Serious psychosocial problems.
Acute signs of severe asthma and potential impending respiratory failure, warranting
admission to an intensive care unit include: 1) Oxygen saturation less than 100%
despite the administration of supplemental oxygen. 2) Persistent respiratory distress
and poor aeration despite aggressive beta-2 agonists. 3) A pCO2 of 40 or greater on a
blood gas. The treatment of severe status asthmaticus bordering on respiratory failure
is controversial. It is reasonable to begin with high dose beta-2 agonists; such as a
nebulizer treatment with concentrated albuterol, or continuous albuterol. In severe
patients, aeration is poor, so inhaling albuterol by itself is usually insufficient.
Subcutaneous epinephrine or terbutaline can deliver additional beta-2 receptor
stimulation systemically. Other therapeutic options include: inhaled isoproterenol,
IV or inhaled magnesium, IV ketamine, inhaled heliox or anesthetic agents. Such
patients should be treated aggressively from the onset to prevent respiratory failure. If
the patient fails to improve and respiratory failure ensues, positive pressure ventilation
should be directed at maintaining oxygenation above 90% saturation if possible.
Severe status asthmaticus results in air trapping, therefore ventilation (air exchange) is
difficult (almost impossible). Although such patients have very high pCO2s because
of air trapping and poor ventilation, the priority should focus on maintaining
oxygenation. Attempting to normalize the pCO2 with aggressive positive pressure
ventilation will increase the likelihood of a pneumothorax which will worsen the
hypoxia. This strategy is known as "permissive hypercapnia" because hypercapnia is
not as deadly as hypoxemia. Permissive hypercapnia is more likely to avoid a
pneumothorax and thus, oxygenation is preserved, improving the overall outcome.
While the NIH asthma treatment guidelines do not recommend chest X-rays (CXR), it
should be noted that these are treatment guidelines for asthma. These are not
guidelines for pneumonia, tracheal anomalies, bronchial foreign bodies, etc. Thus, the
CXR may be necessary in the process of evaluating some patients to be certain that
the patient has asthma and NOT some other condition which can only be identified on
CXR (i.e., to rule out other conditions).
Environmental measures to reduce asthma severity focuses on elimination of
household smoking and the reduction of exposure to dust mite and cockroach
microantigens in the environment. Wrapping mattresses with plastic casings,
conversion of carpeted floors to tile floors, replacing drapes with blinds, and selecting
home furnishings which avoid antigen accumulation, may result in improvement.
Allergy testing and subsequent immunotherapy to desensitize a patient may be
beneficial in some asthmatics.
In summary, asthma is a condition of multiple factors. It can be looked upon as a
syndrome of multiple but related elements. It is basically a chronic condition with
biphasic components which both result in airflow obstruction by different means. The
treatment should take into account the various triggering factors, occupation, age,
psychosocial, and economic factors.
Questions
1. How can you best describe asthma?
2. Can you describe the various medications to treat asthma?
3. Can you describe the parameters that are used to classify severity of
asthma?
4. Describe clinical findings signifying the severity of an acute asthma
exacerbation.
5. Discuss the approach to an asthmatic in relationship to formulating an acute
asthma treatment plan. What questions do you ask, what physical findings do you
look for, and what laboratory parameters are measured?
6. Formulate an asthma maintenance plan.
7. Describe various triggering factors and mechanisms by which they might
exert their action.
8. Describe the immunologic chain of events that ultimately leads to
bronchospasm and inflammation.
9. Discuss the pros and cons of corticosteroid use in children and compare
them with use in adults.
10. How would you convince parents of asthmatics to use medications when
their children are not openly symptomatic?
Answers to questions
1. Asthma is best thought of as a chronic inflammatory condition consisting
of obstruction of the airways of the lung caused by spasms of the smooth muscle
surrounding the airways which, in some cases, can be easily reversed by beta
adrenergic bronchodilators. In other cases, corticosteroids may be necessary to
reverse the airway obstruction by reducing the inflammatory changes responsible for
the airway narrowing. These changes may be caused by a variety of different stimuli.
2. Medications are divided into groups directed towards relaxing bronchial
smooth muscles (relievers) and reversing the inflammation (controllers).
3. This answer can be divided into two parts. The first is used to describe the
degree of severity of the acute asthmatic episode. These would include rate and effort
of respirations, ability to move air through a peak flow meter or spirometer, and
oxygen and carbon dioxide concentration in the arterial blood. The second parameter
involves the sensitivity of the airways (i.e., the chronic severity classification
described in the chapter). Day symptoms, night coughing episodes, peak flow,
coughing with exercise, prolonged coughing after upper respiratory infections, and
coughing with drinking ice-cold beverages help to categorize the severity of asthma.
4. Wheezing may be heard but if the attack is very severe there may be no
wheezing at all (due to poor air exchange). Aeration is a good indicator of acute
severity. Evidence of respiratory distress (retractions, tachypnea) indicates increasing
severity until respiratory failure occurs (at which point, the patient may tire and
exhibit seemingly less respiratory distress). Hypoxemia is also indicative of severity.
Peak flow is typically low for acute exacerbations. For mild cases, cough may be
present at any phase of an asthmatic episode and may be the only sign that
bronchospasm is occurring. A peak flow meter reading before and after a challenge
of inhaled bronchodilator may reveal an increase in the airflow indicating the
presence of bronchospasm.
5. Always consider the triggering event in formulating the treatment plan.
Avoidance of the trigger can be very cost effective. Preventive use of medications
can be very useful such as preemptive use of medication with first sign of a cold.
Analysis of the symptom's response to initial treatment can guide you in up regulating
or down regulating medications. Use of the peak flow meter can serve as an objective
means of adjusting medications. If cough and wheezing occur often and there are
signs/symptoms of chronic asthma, a maintenance plan of daily medication should be
initiated. Efforts should be made to approximate the degree of inflammation in the
airways. This estimation can serve to guide you in the type and dosage of antiinflammatory medications to use. A contingency plan of what medications to use
during an acute episode can be helpful and may help to avoid an unnecessary
emergency visit to the hospital.
6. The asthma maintenance plans are dependent on the patient's severity class
(step 1, 2, 3, or 4). For all "persistent" levels, a daily plan will usually involve a longacting bronchodilator and corticosteroid, LTRA, cromolyn and/or theophylline two to
three times a day. Regular monitoring with peak flow meter readings can help to
determine if the treatment is helping to return the lungs to normal function. A
"rescue" plan using short acting bronchodilators with optional systemic
corticosteroids may be needed for breakthrough wheezing.
7. Allergen exposure is mediated through IgE with resultant immediate and
late phase reactions. A variety of mediators are released and cause a cascade of
immunologic events culminating in tissue edema, increased mucous production, and
sloughing of the epithelial layer of the inner lining of the airways. This affects the
free and easy movement of air to the alveoli, which affects air exchange and causes
atelectasis as the smaller air ways are completely plugged by the thickened mucous.
8. Triggering mast cells cause release of mediators, which can cause
immediate effects on the lung tissue and smooth muscles. Other mediators are formed
and released later and serve primarily to attract inflammatory cells. Some of these
late mediators help to capture the incoming cells. Other mediators recruit epithelial
cells and transform then into participants of the reaction causing them to release more
mediators (biologic amplification).
9. The critical issue of steroids in children is that of linear growth. It is now
well established that the use of inhaled steroids has significantly less effect on growth
than systemic corticosteroids. The length of steroid use (inhaled or systemic), may
have some effect on growth but its effect is temporary and in many studies final
growth of asthmatics is generally no different than in non asthmatics (i.e., catch up
growth occurs if the corticosteroids can be stopped for a period of time long enough
for this to occur). Chronic inflammatory suppression (long term use of inhaled
corticosteroids) improves the long term outcome of asthma (i.e., less severity in the
future).
10. This is where your ability to practice medicine is tested. You need to
educate and persuade the parents that your recommendations are in the best interest of
their child and that it is based on considering the risks against the benefits. This is
ideally done without making the parents feel guilty or intimidated by the potential for
fatal outcomes. While our goal may be to maintain the patient's lifestyle and lung
function, patients may see their goal as getting off medications as soon as possible.
For persistent asthmatics, they should be convinced that this is a chronic disease and
long term medications will be required. Long term use of medications is generally
very safe and not addictive.
References
1. National Institutes of Health. NAEPP Expert Panel Report: Guidelines for
the Diagnosis and Management of Asthma-Update on Selected Topics 2002.
2. Busse WW, Lemamske RF. Advances in Immunology Asthma. New Engl
J Med 2001;344(5):350-363.
3. Kay AB. Advances in Immunology Allergy and Allergic Diseases Part 1.
New Engl J Med 2001;344(1): 30-37.
4. Kay AB. Advances in Immunology Allergy and Allergic Diseases Part 2.
New Engl J Med 2001;344(2):109-114.
5. Marone G, Austen KF, Holgate ST, Kay BA, Lichtenstein LM (eds).
Asthma and Allergic Diseases: Physiology, Immunopharmacology, and Treatment.
1998, San Diego: Academic Press.
6. Barnes PJ, Grunstein MM, Leff AR, Woolcock AJ (eds). Asthma. 1997,
Philadelphia: Lippincott Raven.
7. Pearce N, Beasley R, Burgess C, Crane J. Asthma Epidemiology,
Principles and Methods. 1998, New York: Oxford University Press.
8. Brenner, BE (ed). Emergency Asthma. 1999, New York: Marcel Dekker.
9. Murphy S, Kelly HW (eds). Pediatric Asthma. 1999, New York: Marcel
Dekker.
10. Kotses H, Harver A (eds). Self Management of Asthma. 1998, New
York: Marcel Dekker.
11. Szefler SL, Leung DYM (eds). Severe Asthma: Pathogenesis and
Clinical Management. 2001, New York: Marcel Dekker.
12. Sheffer A (ed). Fatal Asthma. 1998, New York: Marcel Dekker.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
Gastroenteritis:
An 18 month old male is brought to the emergency department with a chief complaint
of diarrhea and vomiting for 2 days. His mother describes stools as liquid and foul
smelling, with no mucous, slime or blood. He reportedly is unable to keep anything
down, vomiting after every feeding, even water. He has about 6 episodes of diarrhea
and 4 episodes of vomiting per day. His mother reports that he is not feeding well and
his activity level is decreased. He seems weak and tired. He has a decreased number
of wet diapers. He attends daycare during the day when he is well. His last weight at
his 15 month check up was 25 pounds (11.4 kg).
Exam: VS T 37.0, P 110, RR 25, BP 100/75, weight 11.3 kg (40th percentile). He is
alert, in mother's arms, crying at times, and looks tired. HEENT: anterior fontanel
closed, minimal tears, lips dry, mucous membranes tacky, no oral lesions or erythema,
TMs normal. His neck is supple. Heart exam reveals mild tachycardia, no murmur.
Lungs are clear. His abdomen is flat, soft, and non-tender with hyperactive bowel
sounds. Testes are descended, non-swollen, non-tender. His diaper is dry. He moves
all his extremities. No rashes are present. His capillary refill time is less than 3
seconds and his skin turgor is slightly diminished.
He is given 40 cc/kg of IV normal saline over two hours in the emergency
department, but when given 30 cc of fluid to drink in the ED, he is unable to hold this
down and he passes another large diarrheal stool. He is then hospitalized for further
management.
Acute gastroenteritis is an ailment that is very common among children. During the
first 3 years of life, a child will likely experience about 1 to 3 acute diarrheal illnesses.
Diarrhea is defined as an increase in fluidity and volume of feces. Nearly all diarrheal
infections are transmitted via the fecal-oral route. Many bacterial etiologies are also
food borne.
When evaluating a child with diarrhea and/or vomiting, several important points and
observations in the history and physical can help to assess severity and determine its
etiology and pathogen involved. Information on the number, volume, and/or fluidity
of stools and emesis should be obtained (1). However, this can be rather cumbersome
if the number of episodes is large since recording the volume of each stool and emesis
is unrealistic and not very helpful clinically, once the number of episodes exceeds 5 to
10. A history of fever, blood or mucus in the diarrhea, foul odor to the diarrhea, a
large quantity of diarrhea or diarrhea for a prolonged duration are suggestive of a
bacterial etiology. Diarrheal "mucus" is not something that is intuitive to most
parents. The best question to ask is whether they have noticed any slimy, gooey,
gelatinous, or mucous-like material in the diarrhea. The presence of mucus in the
diarrhea is generally indicative of sheets of white blood cells in the diarrhea. The
vomiting history should determine whether the vomitus is bilious or bloody (i.e., red
or brown) and whether this is associated with abdominal pain. Parents will often
convey that the vomitus contains mucus, but unlike the mucus in diarrhea, this is
normal gastric mucus that is not helpful clinically. Other important historical items
include: weight loss, dietary (intake) history, ill contacts and travel history. The
physical exam should focus on signs of dehydration, conditions that may suggest an
acute surgical condition and other systemic conditions which may cause these
symptoms. The differential diagnosis of vomiting is extensive including systemic
conditions such as meningitis, increased intracranial pressure, heart failure,
pneumonia, urinary tract infection and many acute surgical conditions such as
appendicitis, intussusception, midgut volvulus, etc. The differential diagnosis of
diarrhea is more limited and is most often due to gastroenteritis.
Many organisms can cause acute infectious diarrhea and vomiting. These include
bacteria, viruses, and parasites. Bacterial etiologies include: Campylobacter, E. coli,
Staph aureus, Salmonella, Shigella, Vibrio, Yersinia and a few others. Viral
gastroenteritis is by far more common. Amebic and parasitic etiologies are not very
common in the United States, but such cases are treatable, making the identification
of the pathogen essential.
Lab tests available include: CBC, stool Wright stain, stool culture, stool Rotazyme,
serum electrolytes and glucose. In most instances, no laboratory studies are required.
A CBC might raise the clinical suspicion of a bacterial etiology if a very high band
count is present; however, this may also occur in viral etiologies, therefore it is not
very helpful in most instances. A positive stool Wright stain identifies WBCs in the
stool. This is suggestive of a bacterial etiology (similar to the history or observation
of mucus in the stool), but since it is unable to determine the specific pathogen, it does
not help clinicians in determining whether antibiotics are indicated. Thus, in most
instances of gastroenteritis, a Wright stain is not helpful. A stool culture can be
obtained by swabbing a diarrhea sample or by inserting a swab into the rectum, then
rotating it to obtain organisms from the rectal mucosal surface. This latter method is
called a rectal swab and it has a higher yield for identifying enteric pathogens, which
are more likely to be found on the rectal mucosal surface than in the diarrhea itself.
Thus, if a stool culture is to be obtained, it should be done via a rectal swab. A stool
Rotazyme is a rapid test which identifies the presence of rotavirus in the diarrhea. A
positive Rotazyme negates the need for a stool culture and antibiotic therapy. Serum
electrolytes and glucose may be helpful in determining the degree of electrolyte
imbalance, metabolic acidosis and hypoglycemia.
Campylobacter
Campylobacter is a major cause of diarrhea in the world. Many species have been
identified as enteropathogens, but C. jejuni and C. coli are the two predominant
species causing acute diarrhea in humans. C. jejuni is the most commonly
documented bacterial cause of diarrhea. Transmission occurs by the fecal-oral route,
through contaminated food and water or by direct contact from infected animals and
people. The main source of C. jejuni and C. coli infection in humans is poultry,
although dogs, cats, and hamsters are also potential sources. Outbreaks of diarrhea
caused by C. jejuni and C. coli have been associated with consumption of
undercooked poultry, red meat, unpasteurized milk and contaminated water. In
industrialized nations, C. jejuni mainly affects children younger than 5 years of age,
and individuals 15-29 years old. In temperate climates, infections occur mostly
during the warm months, and in tropical climates, the incidence is greater during the
rainy season. Prodromal symptoms begin after an incubation period of 1-7 days.
These include fever, headache, chills and myalgia. Diarrhea accompanied by nausea,
vomiting, and crampy abdominal pain occurs within 24 hours. Stools vary from loose
and watery, to grossly bloody. Abdominal pain affects more than 90% of patients
older than 2 years, and if severe enough, may mimic appendicitis. The clinical
presentation may also mimic inflammatory bowel disease. Gradual resolution is to be
expected, but in 20% of cases, diarrhea can last longer than 2 weeks. The variation in
clinical presentation of Campylobacter diarrhea makes clinical diagnosis difficult. In
order to differentiate it from other causes of colitis such as Salmonella, Shigella, E.
coli 0157:H7, one has to rely on microbial diagnosis (2).
Rehydration and correction of electrolyte abnormalities are the mainstay of treatment.
The use of antimicrobial therapy remains a controversial issue since resolution
without antibiotics usually occurs, but early initiation of antibiotic therapy can shorten
the duration of infectivity (i.e., the excretion of the organisms), and the duration of
symptoms. Erythromycin is the antibiotic of choice for the treatment of C. jejuni
enteritis. Since it is not possible to reliably identify Campylobacter as the etiology
prior to a positive culture, erythromycin is generally not prescribed until several days
after a culture has been obtained. A notable exception might be when diarrhea
develops in a contact of an individual who was known to have Campylobacter
gastroenteritis.
C. jejuni has also been associated with septicemia, abortion and Guillain-Barre
syndrome. C. pylori, now called Helicobacter pylori, does not cause diarrhea, but is
associated with peptic ulcer disease. C. fetus rarely causes diarrhea, but is recognized
as a cause of fever, bacteremia in immunocompromised hosts, and spontaneous
abortion.
Escherichia coli
E. coli is one of the most common causes of bacterial diarrhea in humans worldwide.
Normal bowel flora consists mostly of E. coli; however, some strains of E. coli are
pathogenic.
Several categories of
E. coli cause diarrhea:
EHEC
(enterohemorrhagic), ETEC (enterotoxigenic), EIEC (enteroinvasive), EPEC
(enteropathogenic), and EAEC (enteroaggregative).
EHEC causes a hemorrhagic colitis syndrome manifested by bloody diarrhea without
fever. Illness is characterized by abdominal pain with diarrhea that is initially watery,
but becomes blood streaked or grossly bloody. E. coli O157:H7 has most commonly
been associated with this syndrome, which can also be caused by other strains of
EHEC which produce large quantities of a potent cytotoxin. The cytotoxin produced
by E. coli is called Verotoxin 1, and is virtually identical to the shigatoxin. Unlike
shigellosis or EIEC disease, fever is uncommon in this diarrheal illness. The strains
of E. coli producing this potent cytotoxin, are important because 5% to 8% of
symptomatic patients go on to develop hemolytic uremic syndrome (HUS).
ETEC typically causes explosive diarrhea, accompanied by nausea, vomiting,
abdominal pain, with little or no fever. Symptoms resolve in a matter of days, but
these organisms can have a major effect on the hydration status of infants. ETEC is
often responsible for the syndrome of "traveler's" diarrhea, since it is more common in
non-industrialized countries (note the "T" in enteroToxigenic and Traveler's).
EIEC causes a dysentery-like illness or watery diarrhea caused by an enterotoxin
called the EIEC enterotoxin. This illness is clinically indistinguishable from shigella
dysentery, and is characterized by fever, abdominal pain, tenesmus, watery or bloody
diarrhea.
EPEC rarely causes diarrhea in older children and adults, but has been incriminated in
sporadic and epidemic diarrhea in infants and children in the first 2 years of life. This
can be remembered as pediatric diarrhea (note the "P" in enteroPathogenic and
Pediatric). The diarrhea is non-bloody, and contains mucus. These organisms can
cause prolonged diarrhea, especially in the first year of life, a feature that in not
shared by the ETEC, EIEC, or EHEC.
EAEC have been associated with acute and chronic diarrhea in developing countries.
They can cause significant fluid losses. Like EPEC, these organisms can cause a
prolonged diarrhea, and severe abdominal pain, lasting 2-4 weeks.
Management of fluid and electrolyte losses should be the focus of treatment.
Early refeeding (within 8-12 hours or initiation of rehydration) should be encouraged,
because a prolonged delay in feeding can lead to chronic diarrhea and dehydration.
The decision to treat with antibiotics is difficult, because of the lack of a rapid
diagnostic test. EPEC and ETEC respond well to treatment with trimethoprimsulfamethoxazole, but antimicrobial treatment of EHEC organisms may increase the
risk of hemolytic uremic syndrome (1). Thus, it is preferable to withhold antibiotics
until a culture proven etiologic indication for antibiotics is determined.
Staphylococcus aureus
S. aureus is a major cause of food poisoning. Symptoms begin within 1-6 hours after
ingestion contaminated with preformed S. aureus heat-stable enterotoxins. Symptoms
include nausea, vomiting, abdominal pain, diarrhea without fever, and last less than
12 hours (1).
The term "food poisoning" describes a phenomenon in which bacterial contamination
of food results in toxin production by the bacteria. This toxin ingestion is what causes
the acute symptoms. Typically, the onset after exposure is rapid (i.e., no incubation
period is required) and resolution is relatively rapid. Food poisoning should be
distinguished from food borne infection, in which the latter is due to contamination of
food which is ingested and symptoms develop several days later after a period of
incubation.
Salmonella
Salmonella is one of the most frequently reported causes of food borne outbreaks in
the United States. Three main species are identified: S. cholerae-suis, S. typhi
(known as typhoid), and S. enteritidis. Salmonella gastroenteritis occurs throughout
life, but is most common in the first year of life. Many outbreaks of S. enteritidis
have been associated with ingestion of contaminated eggs. For clinical disease to
occur, 10,000 to 100,000 viable organisms must be ingested. The incubation period
for salmonella gastroenteritis is 6 to 72 hours. The major virulence trait of salmonella
species, especially S. typhi, is their ability to invade the intestinal epithelium.
Nontyphoidal salmonella can also display this trait, but most are associated with
watery diarrhea.
Salmonella causes several clinical syndromes:
1) acute
gastroenteritis, 2) a subacute or prolonged carrier state, 3) enteric fever, bacteremia or
both, and 4) dissemination with localized suppuration (i.e., abscesses), osteomyelitis,
or meningitis (3).
The most common manifestation of disease caused by salmonella is gastroenteritis.
The most prominent symptom is diarrhea, which is usually self-limited, lasting 3-7
days. However, the diarrhea can range from a few stools, to profuse bloody diarrhea
to a cholera-like syndrome. Abdominal cramps and fever are usually present (3).
The diagnosis is made by stool culture. Antimicrobial agents such as ampicillin,
chloramphenicol, trimethoprim-sulfamethoxazole and some third generation
cephalosporins can be used in patients with severe and progressing disease. Initiation
of empiric antibiotic treatment is not indicated unless: 1) the infant is very young, 2)
the patient is immunocompromised, or 3) sepsis or disseminated infection is
suspected.
Shigella
Infection by shigella is rare in the first 6 months of life, but is common between 6
months and 10 years, and is most common in the 2nd and 3rd year of life. There are 4
major serogroups of Shigella: S. sonnei, S. flexneri, S. boydii, and S. dysenteriae. S.
sonnei and S. flexneri are more common causes of diarrhea in the U.S. and Europe.
Infection occurs mostly during the warm months in temperate climates, and during the
rainy season in tropical climates. Transmission is mainly via person-to-person contact
and by contamination of food and water. Only a small inoculum of shigella is
required to cause illness (as few as 10 organisms). After ingestion, an incubation
period of 12 hours to several days follows, before the development of symptoms.
Shigella organisms invade the epithelial cells of the colon, causing colitis. Infected
individuals may present with: 1) asymptomatic excretion, 2) enterotoxin-like
diarrhea, 3) bacillary dysentery (bloody diarrhea), 4) arthritis similar to Reiter's, 5)
HUS after infection with S. dysenteriae (1).
Diarrhea is initially watery and of large volume, and develops into frequent small
volume, bloody and mucoid stools. Other clinical features include, vomiting, severe
abdominal pain, high fever, and painful defecation. As many as 40% of children with
bacillary dysentery will develop neurologic findings. Seizures, headache, confusion,
lethargy, and/or nuchal rigidity may be present before or after the onset of diarrhea.
In very young and malnourished patients, sepsis and disseminated intravascular
coagulation can develop as complications. When sepsis occurs, the mortality rate is
high. In those infected with S. dysenteriae serotype 1, hemolytic anemia and
hemolytic uremic syndrome are common complications caused by shigatoxin. Post
infectious arthritis occurs 2 to 5 weeks after dysenteric illness, and is seen
characteristically in patients with HLA-B27 (1).
Stool culture (rectal swab) is the gold standard for diagnosis. The presence of fecal
leukocytes, fecal blood, and a CBC leukocytosis with left shift can help support a
presumptive diagnosis of bacterial gastroenteritis. Fluid and electrolyte correction
and maintenance should be the initial focus of treatment. Drugs that delay intestinal
motility (Lomotil, loperamide) should be avoided. Empiric antibiotic treatment of all
children strongly suspected of having shigellosis is highly encouraged, and should be
initiated as soon as possible. However, other bacterial etiologies may be worsened by
antibiotic treatment, so it may be preferable to await culture results.
Vibrio cholera and parahaemolyticus
Vibrio species are common causes of diarrhea worldwide. V. cholera serogroups O1
and O139 have been responsible for cholera epidemics in many developing countries.
These serogroups produce profuse watery diarrhea (known as rice water stools) after
adhering to and multiplying on small intestinal mucosa. They cause diarrhea by
producing several toxins. The most important toxin is the cholera toxin which is a
heat stable enterotoxin. V. parahaemolyticus is a common marine organism found in
water, shellfish, fish, and plankton. It is generally an uncommon cause of diarrhea,
but people who consume raw shellfish and seafood are at higher risk. Infected
individuals experience diarrhea, abdominal cramps, nausea, and less frequently,
vomiting, headache, low grade fever and chills (1).
Yersinia enterolitica
Y. enterolitica is a gram negative bacillus that appears to be a common cause of
gastroenteritis among children in Europe and Canada, but is uncommon in the United
States. Outbreaks have been associated with ingestion of contaminated milk or food.
The clinical manifestations vary depending on the age of the involved person. It
usually causes acute gastroenteritis in younger children, and mesenteric adenitis in
older children. Most infections occur in children 5 to 15 years of age, and incidence
is greater during the winter months. Children younger than 5 years old usually have
self-limited gastroenteritis, lasting from 2 to 3 weeks. Symptoms include diarrhea
accompanied by fever, vomiting, and abdominal pain. The abdominal pain is colicky,
diffuse, or localized to the right lower abdomen. Stools may be watery, containing
blood or mucus, and fecal leukocytes are commonly present. Children older than 6
years often present with abdominal pain associated with mesenteric adenitis that
mimics acute appendicitis. Intussusception secondary to Y. enterolitica has also been
described in children.
While isolation of Y. enterolitica from stool specimens is difficult, it is readily
isolated from blood or lymph nodes. Serologic diagnosis can be obtained with
enzyme-linked immunosorbent assays. Antibodies are detectable from 8-10 days
after the onset of clinical symptoms, which persist for several months.
Y. enterolitica gastroenteritis is usually self-limited, therefore antibiotic therapy is
only indicated for complicated infections, and compromised patients. It is usually
susceptible to trimethoprim-sulfamethoxazole, aminoglycosides, tetracycline,
chloramphenicol, and third generation cephalosporins. It is resistant to penicillin,
ampicillin, carbenicillin, erythromycin, and clindamycin (4).
Other bacteria
Other bacteria that cause an acute diarrheal illness include Bacillus cereus,
Aeromonas hydrophila and Plesiomonas shigelloides. Clostridium difficile is
associated with pseudomembranous colitis, and Clostridium perfringens can cause a
short duration food poisoning syndrome.
Viral gastroenteritis
Diarrhea and vomiting caused by viruses are usually self-limited. It usually manifests
as watery diarrhea, without blood or mucus. The most common accompanying
symptom is vomiting. Other symptoms include abdominal cramps, nausea,
headaches, myalgias and fever. Four viral groups are regarded as medically important
causes of acute gastroenteritis (rotaviruses, astroviruses, enteric adenoviruses, and
calciviruses). They are all spread largely via the fecal-oral route.
Rotavirus accounts for 82,000 hospitalizations and 150 deaths per year in the United
States. It is a very common cause of acute gastroenteritis in infants and children,
responsible for over 50% of cases of acute diarrhea in children. Rotavirus causes
severe illness in children 6-24 months. Most children (more than 90%) have been
exposed to rotavirus by their 3rd birthday. It is the most common cause of diarrhea in
infants and children in the winter months in colder climates, and is responsible for 3550% of hospitalization for infants and children with acute diarrhea. Clinical features
range from asymptomatic infection, to diarrhea preceded by severe vomiting. The
incubation period is 2-4 days, and viral shedding occurs from a few days before, to 10
days after the onset of illness. Newborns tend to have asymptomatic infections in the
first few months of life, because transplacental antibodies and breastfeeding are
protective. Clinical symptoms in infants and children usually consist of fever, abrupt
onset of vomiting and watery diarrhea. One third of children will also have a
concurrent respiratory infection. The diagnosis can be made by a number of enzyme
immunoassays (Rotazyme) and latex agglutination tests with good specificity and
sensitivity. Stools collected early in the course of the illness are more likely to
contain virus, than those collected 8 or more days after the onset of illness. Treatment
is directed toward correction of dehydration with oral fluid replacement.
Breastfeeding is the most important and available preventive strategy, because human
colostrum contains rotavirus antibodies (5).
Adenovirus commonly causes a wide range of human diseases, including
conjunctivitis, pneumonia, and upper respiratory infections. A subgroup of
adenovirus (enteric adenovirus) has been found to cause acute gastroenteritis, lasting
4 to 20 days. After rotavirus, enteric adenovirus is the next most common cause of
viral gastroenteritis in infants and children, accounting for 5% to10% of
hospitalizations for acute gastroenteritis in children. They have been linked to
outbreaks in child care centers, and asymptomatic excretion can occur. Most episodes
occur in children less than 2 years. Infection increases in the summer months.
Symptoms are indistinguishable from those associated with rotavirus but are less
severe. The diagnosis is a presumptive one. Research laboratory confirmation can be
made by solid phase immunoassays, electron microscopy, and viral culture but these
studies are not routinely available. As with rotavirus, treatment is aimed at replacing
fluid losses, and at correcting electrolyte abnormalities (5).
Caliciviridae primarily infect infants and young children. Human caliciviruses
(HuCVs) have been associated with outbreaks from contaminated food or water in
restaurants, schools, hospitals, summer camps and cruise ships. They are divided into
4 genera: Norwalk-like calciviruses (which causes illness mostly in adults), Sapporolike calcivirus (which primarily cause pediatric gastroenteritis), rabbit-like calcivirus,
and swine-like calicivirus. Hepatitis E, which was previously classified as a
calcivirus, is no longer classified in this family.
The gastroenteritis is
indistinguishable from that of rotavirus. The incubation period is 12 hours to 4 days.
Excretion lasts 5-7 after the onset of symptoms, and can continue for 4 days after the
resolution of symptoms (5). The diagnosis is presumptive, but epidemiologic and
research confirmation of these etiologic agents can be made by electron microscopy.
Astrovirus infection is believed to occur very commonly, since 80% or more adults
have antibodies against the virus. They cause watery diarrhea, especially in children
younger than 4 years. Symptoms are mild, consisting of fever and malaise, followed
by watery diarrhea. Vomiting is uncommon. The incubation period is 3 to 4 days,
and excretion typically lasts for 5 days after onset of symptoms (5).
Protozoan and parasitic gastroenteritis
Several protozoans cause diarrhea:
Giardia lamblia, Entamoeba histolytica,
Cryptosporidium parvum, Isospora belli, Microsporidium species, and Cyclospora
cayetanensis. Within the intestinal tract, 3 organisms, namely Giardia lamblia,
Entamoeba histolytica, and Cryptosporidium parvum are the most important.
Infection with E. histolytica occurs more frequently in tropical countries, especially in
areas with poor sanitation. The life cycle of E. histolytica has 2 stages: the
trophozoite which is found in diarrheal stools, and the cyst, which is more
predominant in formed stools. The cysts are transmitted primarily by the fecal-oral
route in contaminated food and water. The minimum incubation period is 8 days, but
ranges up to 95 days. The clinical symptoms that occur in patients with amebiasis
consist of: 1) Intestinal amebiasis, with the gradual onset of colicky abdominal pain
and frequent bowel movements, tenesmus, and flatulence. 2) Amebic dysentery,
characterized by profuse diarrhea containing blood, mucus, and constitutional signs
such as fever and dehydration. 3) Hepatic amebiasis, presenting with an amebic
abscess within the liver often without diarrhea. 4) Asymptomatic infection. E.
histolytica can usually be identified in stool samples, or acute infection can be
confirmed by serology. Metronidazole is the treatment of choice.
Giardia lamblia is a flagellated protozoan that is a major cause of diarrhea, especially
in patients who travel to endemic areas. It is the most common intestinal protozoan
found in the United States. Children seem to be more susceptible to Giardia than
adults. Like E. histolytica, the life cycle consists of 2 stages: the trophozoite (motile
form), and the cyst. IgA deficiency and hypogammaglobulinemia predispose patients
to symptomatic infection. The clinical manifestations are foul-smelling diarrhea, with
nausea, anorexia, abdominal cramps, bloating, belching, flatulence, and weight loss.
Abdominal distention and cramps can last for weeks to months. The illness is usually
self-limited, lasting 2 to 6 weeks, but may become chronic. Chronic symptoms can
include fatigue, nervousness, weight loss, steatorrhea, lactose intolerance, and growth
retardation. The easiest way to diagnose Giardia is by identifying cysts in a stool
specimen. However, these specimens are frequently falsely negative. The diagnosis
can also be made by antigen detection tests, endoscopic examination of the upper
small intestine, by mucosal biopsy or by collection of jejunal contents. The treatment
of choice for both symptomatic and asymptomatic patients is furazolidone or
metronidazole. An alternative drug is quinacrine (6).
Cryptosporidium organisms are small protozoans that are a common cause of enteric
infection worldwide. The oocyst form of cryptosporidium is found in feces, and is the
infective form. Cryptosporidium parvum is thought to be the cause of infection in
humans. Cryptosporidium causes cryptosporidiosis mainly in immunocompromised
patients, infecting approximately 3% to 4% of patients with AIDS in the United
States. Another high risk group for acquiring infection with this organism, are
children 6 to 24 months old. It is acquired by fecal-oral transmission, and has an
incubation period of 2 to 14 days. Because the oocyst is highly stable in the
environment, contaminated drinking water, swimming pools and apple cider are major
sources of outbreaks. Several outbreaks of diarrhea in day care centers have been
attributed to cryptosporidium. In the United States, 13% of children less than 5 years
old, 38% of those 5 to 13 years of age, and 58% of adolescents 14 to 21 years old are
seropositive for C. parvum. Symptoms are self-limited in immunocompetent patients,
consisting of watery nonbloody diarrhea, accompanied by vomiting, flatulence,
abdominal pain, myalgias, anorexia, weight loss, and low-grade fever. Symptoms last
an average of 9 days in immunocompetent patients, but can last months in
immunocompromised hosts, causing large fluid losses, profound malabsorption and
weight loss. The diagnosis of cryptosporidiosis is made by demonstrating the
presence of cryptosporidium oocysts in stool specimens. Patients with diarrhea who
attend day care centers, those with AIDS or other immunodeficiencies are at higher
risk. There is no specific antimicrobial therapy for cryptosporidiosis (7).
Dehydration
Fluid losses resulting from acute vomiting and diarrhea can lead to dehydration.
Diarrhea is the most common cause of dehydration in infants and children, and is a
leading cause of death worldwide in children less than 4 years of age. Quantifying the
degree of fluid loss by history and the amount and type of fluid intake can help to
determine dehydration severity and the risk of electrolyte imbalance. The amount of
urine output and the presence or absence of tears as well as the presence of
documented weight loss, can help determine the severity of dehydration present.
Other important aspects of the history include the presence of fever, sweating and
hyperventilation, which may cause insensible losses, contributing to the degree of
dehydration.
The vital signs can offer clues on the degree of dehydration present. Tachycardia can
indicate moderate dehydration, whereas hypotension is a late sign of severe
dehydration. The absence of tachycardia cannot be used to rule out dehydration. An
increase in the respiratory rate is associated with a higher degree of dehydration,
whereas in mild dehydration, the respiratory rate is normal. Attention should be paid
to the child's overall appearance and mental status (alert, irritable, tired-appearing,
poorly responsive, unresponsive). Other pertinent exam findings include the
fontanelle (sunken or not), the eyes (presence or absence of tears, sunken or not), the
mouth (dry lips, tacky or sticky mucous membranes), the skin (cool or warm, skin
turgor). Measurement of the capillary refill time is a variable and unreliable indicator
of dehydration. It should be performed in a warm room. Light pressure is applied to
the finger nailbed. And the time from blanching to restoration of color to the nailbed
is measured. It may be preferable to assess capillary refill centrally (over the chest or
forehead) and peripherally (fingers), so that the two can be compared. A delay of less
than 2 seconds is normal. Delays of 2 to 3 seconds may indicate moderate
dehydration, and more than 3 seconds in delay may indicate severe dehydration.
Most children with clinically significant dehydration, will have 2 of the following 4
clinical findings: 1) capillary refill greater than 2 seconds, 2) tacky mucous
membranes, 3) no tears, and 4) ill appearance (8).
Management
The decision to hospitalize or to attempt outpatient management will be based on the
clinical findings, combined with a history of fluid intake, the frequency of urination,
assessment of concurrent stool losses and the response to therapy.
Once a child is presumed to be dehydrated, the degree of dehydration needs to
be determined. Acute weight loss can be used to determine the degree of dehydration,
but accurate baseline weights in growing children are almost never known. Clinical
criteria can be used to estimate degrees of dehydration. Mild dehydration is 5% or
less, moderate is about 10%, and severe dehydration is about 15% or greater. This
classification is relative and not well standardized. If severe dehydration or
uncompensated shock is present, the patient should be immediately treated with an IV
fluid infusion (20 cc/kg of normal saline or lactated Ringer's) to restore the
intravascular volume. It is likely that more than one fluid bolus may be necessary to
restore the patient's intravascular volume, since 20 cc/kg only corrects 2% of the body
weight. Therefore, the patient should be reassessed after each fluid bolus (8).
Children with mild to moderate dehydration can be initially treated with oral
rehydration. Contraindications to oral rehydration therapy (ORT) include severe
dehydration, intractable vomiting, and severe gastric distention. Children who had
initially received IV fluid infusions for severe dehydration who now feel well enough
to take oral fluids should also be considered for oral rehydration. Oral rehydration
therapy (ORT) can be as effective as IV therapy. It is noninvasive and inexpensive.
The AAP recommends that rehydration solutions contain 70-90 mEq/L of sodium, 20
mEq/L of potassium, and 2.0-2.5 grams/dL glucose. Examples of rehydration
solutions (ORS) are: Rehydralyte (70mEq/L Na, 20 mEq/L K, 2.5% glucose, and the
WHO solution (90m Eq/L Na, 20 mEq/L K, 2% glucose). ORT should initially be
given in small, frequent volumes, 5 to 20 cc every 5-10 minutes, and advanced slowly
to approach 5 cc/min. The degree of dehydration and the presence of ongoing losses
dictate the volume of fluids to be administered. If the degree of dehydration is mild
(3-5%), the volume of ORS administered should be 50 cc/kg (i.e., 5% of the body
weight) over 4 hrs. Those with significant dehydration (5-10%) should received 100
cc/kg (i.e., 10% of the body weight) of ORS over 4 hours. In either case, an
additional 10 cc/kg should be given for each diarrheal stool seen. Once rehydration is
complete, maintenance fluid is given. Examples of maintenance oral solutions are:
Pedialyte and Infalyte, containing 45-50 mEq/L Na, 2-2.5 mEq/L K, 1.5-2.5% glucose
(9).
Patients with mild dehydration can potentially be managed without laboratory
analysis. However, in moderate or severe dehydration, laboratory studies should be
obtained to look for electrolyte abnormalities of to measure the degree of metabolic
acidosis.
Children who are severely dehydrated and those who cannot retain oral fluids because
of intractable vomiting should be hospitalized and treated with IV fluid. Once the
initial resuscitation phase is completed, replacement IV therapy should be instituted,
taking into account fluid and electrolyte deficits as well as ongoing losses. Usually,
half of the replacement therapy in addition to the maintenance fluid requirement is
given over the first 8 hours, and the second half is given over the next 16 hours.
However, patients with hypernatremic dehydration (serum sodium >150mEq/L)
require special intervention. After initial management with normal saline or lactated
Ringer's, the replacement fluid is given more slowly, over 48 hours or more. This is
done because rapid correction of hypernatremia can result in acute brain swelling,
brain herniation, and death. Therefore, care should be taken to avoid dropping the
serum sodium by more than 15mEq/L per 24 hours.
Once a child is adequately rehydrated, the question of when to start feedings arises. It
was previously perceived that a period of "gut rest" should follow rehydration of
patients with acute gastroenteritis. However, numerous trials have shown no
advantage to this strategy. The concept of early refeeding is replacing the old concept
of "gut rest". Numerous trials have shown that early feeding of age-appropriate foods
results in faster recovery. Breast fed infants should continue nursing despite diarrhea.
Following rehydration, children with mild diarrhea who drink milk or formula can
tolerate full strength feedings. The traditional BRAT diet (bananas, rice, applesauce,
toast), although acceptable, should be considered to be a concept representative of a
bland diet rather than a specific diet. Controlled clinical trials have shown that
starches, complex carbohydrates (rice, wheat, bread, potatoes, cereals), soups, fresh
fruits and vegetables, yogurt, and lean meats are better choices, and well tolerated (9).
Fatty foods, juices, teas, sweetened cereals, soft drinks, are poor choices, and should
be avoided. Some patients may benefit from lactose-free or low-lactose formulas.
Most pediatricians and experts recommend against using anti-diarrheal agents such as
Imodium (loperamide), Pepto-Bismol (bismuth subsalicylate), and Kaopectate. This
is more of a precaution since many studies do show some beneficial effects from these
medications in patients with mild diarrhea. However, patients with mild diarrhea will
get better on their own so these medications are usually not necessary. For young
children with severe gastroenteritis, there is insufficient data to confirm the benefit
and safety of these medications, which is why they cannot be recommended routinely
at this time.
Questions
1. Which diarrhea causing organism may be also cause neurologic symptoms?
2. What is the most common viral cause of acute gastroenteritis, and what are
its associated symptoms?
3. How is Giardia lamblia most easily diagnosed and how is it treated?
4. List 4 physical signs of dehydration in children?
5. How are children with mild dehydration initially treated?
6. How are children with severe dehydration initially treated?
References
1. Pickering LK, Cleary TG. Chapter 52-Approach to patients with
gastrointestinal tract infections and food poisoning. Feigin RD, Cherry JD (eds).
Textbook of Pediatric Infectious Diseases, 4th edition. 1998, Philadelphia: W.B.
Saunders, pp. 567-589.
2. Ruiz-Palacios GM, Pickering LK. Chapter 163- Campylobacter and
Helicobacter. In: McMillan JA, DeAngelis CD, Feigin RD, et al (eds). Oski's
Pediatrics, 3rd edition. 1999, Philadelphia: Lippincott Williams & Wilkins, pp. 953956.
3. Chacon-Cruz E, Pickering LK. Chapter 179- Salmonella infections. In:
McMillan JA, DeAngelis CD, Feigin RD, et al (eds). Oski's Pediatrics, 3rd edition.
1999, Philadelphia: Lippincott Williams & Wilkins, pp. 1002-1006.
4. Cleary TG, Gomez HF. Chapter 187- Yersenia Enterolitica. In: McMillan
JA, DeAngelis CD, Feigin RD, et al (eds). Oski's Pediatrics, 3rd edition. 1999,
Philadelphia: Lippincott Williams & Wilkins, pp. 1042-1044.
5. Matson DO, Pickering LK, Douglas K. Mitchell DK. Chapter 212-Viral
Gastroenteritis. In: McMillan JA, DeAngelis CD, Feigin RD, et al (eds). Oski's
Pediatrics, 3rd edition. 1999, Philadelphia: Lippincott Williams & Wilkins, pp.
1147-1150.
6. Klish WJ. Chapter 224- Giardia Lamblia. In: McMillan JA, DeAngelis
CD, Feigin RD, et al (eds). Oski's Pediatrics, 3rd edition. 1999, Philadelphia:
Lippincott Williams & Wilkins, pp. 1176-1177.
7. Hughes WT. Chapter 223- Cryptosporidiosis. In: McMillan JA,
DeAngelis CD, Feigin RD, et al (eds). Oski's Pediatrics, 3rd edition. 1999,
Philadelphia: Lippincott Williams & Wilkins, pp. 1174-1176.
8. Shaw KN. Chapter 18- Dehydration. In: Fleisher GR, Ludwig S, Henretig
FM (eds). Textbook of Pediatric Emergency Medicine, 4th edition. 2000,
Philadelphia, Lippincott Williams & Wilkins, pp. 197-201.
9. American Academy of Pediatrics. Practice parameter: the management of
Acute Gastroenteritis in Young Children. Pediatrics 1996;97:424-435.
10. Sharifi J. Ghavami F. Oral rehydration therapy if severe diarrhea
dehydration. Clin Pediatr 1984;23:87-90.
11. Bezerra JA, Stathos TH, Duncan B, et al. Treatment of infants with acute
diarrhea: What's recommended and what's practiced. Pediatrics 1991:90:1-4.
12. Reis EC. Goepp JG, Katz S, et. al. Barriers to the use of oral rehydration
therapy. Pediatrics 1994:93:708-711.
13. Feigin RD, Kaplan JB. Chapter 156-Aeromonas. In: McMillan JA,
DeAngelis CD, Feigin RD, et al (eds). Oski's Pediatrics, 3rd edition. 1999,
Philadelphia: Lippincott Williams & Wilkins, pp. 932-933.
Answers to questions
1. Shigella.
2. Rotavirus. It causes fever, vomiting, and watery diarrhea.
3. The diagnosis can be made by antigen detection, identifying cysts in the
stool, endoscopy or examination of jejunal contents. It is treated with metronidazole
or furazolidone.
4. Sunken fontanelle, absence of tears, sunken eyes, sticky/tacky oral mucosa,
delayed capillary refill, reduced skin turgor, inactivity/lethargy, tachycardia,
hypotension.
5. With oral rehydration, small frequent volumes 5-20cc every 5-10 minutes,
advanced slowly.
6. With IV fluid infusion of normal saline or lactated Ringer's at 20cc/kg.
Oral rehydration with ORS is commonly employed in other countries.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
Hepatitis:
This is a 14 year old Caucasian patient who comes to your office with a chief
complaint of nausea, poor appetite, and fever for one week. Her fever has been
between 101 and 102 degrees, and occurs every day. She had two non-bloody, nonbilious episodes of emesis in the last two days and also has abdominal pain on her
right side below the ribs, which has been getting worse. Her urine color is darker than
normal, although she has not been drinking much fluid. She denies any coughing,
URI symptoms, diarrhea, and dysuria. She is not sexually active and denies drug or
alcohol use. She is not taking any medications other than ibuprofen for her fever.
Exam: T38.4, P85, R16, BP 100/70. She has a 2.3 kg (5 pound) weight loss since her
last visit 6 months ago. She appears tired and ill-appearing, but is cooperative with
her examination. Her skin is jaundiced and there is scleral icterus. Her liver edge is
palpable 5 cm below the right costal margin, and is moderately tender. There is no
splenomegaly. The rest of her examination, including ophthalmologic, cardiac,
pulmonary, and neurological systems, is normal. She has no lymphadenopathy.
Laboratory data: Normal CBC and reticulocyte count. Electrolytes, BUN, and
creatinine normal. AST 500, ALT 550, alkaline phosphatase 500, GGT 50, total
bilirubin 13.5 (direct fraction 5.0). Prothrombin time is 14.0 seconds (prolonged).
In light of her presentation, additional blood work is sent for anti-HAV IgM, HBsAg,
anti-HBc, and HCV. On further questioning, it is discovered she ate at a restaurant
one month ago where a worker was found to have hepatitis A. Her blood work later
returns positive for hepatitis A.
The liver performs many essential functions. It is the first to receive blood from the
intestines through the portal vein. It stores vitamins B12, A, D, E, and K. It also
stores glucose in the form of glycogen, or converts it into fatty acids. It deaminizes
amino acids into ammonia, which is then converted into urea. It manufactures
proteins such as albumin, prothrombin, fibrinogen, transferrin, and glycoprotein from
amino acids. The cytochrome P-450 system is responsible for the detoxification of
many different compounds (e.g., drugs), and is important in the metabolism of steroid
hormones and fatty acids. It also synthesizes bile and plays an important role in lipid
metabolism. It excretes bilirubin and biliverdin formed from heme in red blood cells
from the reticuloendothelial system in different parts of the body (1). Therefore,
diseases that damage the liver can have a very detrimental effect on the body. This
chapter will discuss some of the diseases that affect the liver, focusing on viral
hepatitis.
Hepatitis is an inflammation of the liver and can be due to many different causes.
Although viral hepatitis is well known, other diseases include autoimmune causes
such as systemic lupus erythematosus, drug-induced causes such as isoniazid, and
metabolic disorders such as Wilson disease, alpha-1-antitrypsin deficiency,
tyrosinemia, Niemann-Pick disease type 2, glycogen storage disease type IV, cystic
fibrosis, galactosemia, and bile acid biosynthetic abnormalities (2). The anatomy and
physiology of the liver is complex and outside the scope of this chapter, although its
basic concepts are important to understand the pathophysiology of liver disease. The
common theme is that there is injury or death to the hepatocytes. When this occurs,
enzymes in these cells are released, which include the aminotransferases (aspartate
aminotransferase and alanine aminotransferase), alkaline phosphatase, and gammaglutamyl transpeptidase (GGT). It should be noted that these aminotransferases are
also located in heart and skeletal muscle tissues, although alanine aminotransferase
(ALT) is more specific to the liver than aspartate aminotransferase (AST) and has a
longer plasma half-life (which makes an elevation of AST indicative of early hepatic
damage). Alkaline phosphatase, besides being found in the liver, is also present in
kidney, bone, placenta, and intestine. GGT is found in biliary epithelia and
hepatocytes, and is therefore a more specific marker for liver disease (3). A
misnomer is that liver function tests (LFT's) consists of measuring the levels of
aminotransferases, alkaline phosphatase, and GGT. However, these intracellular liver
enzymes are not indicative of liver function, but rather damage to the liver. As was
mentioned earlier, the liver has many functions, such as the production of proteins
from amino acids, gluconeogenesis and glycogenolysis, and the excretion of bilirubin.
Therefore, damage to the hepatocytes will result in decreased production of proteins,
notably albumin, prothrombin, fibrinogen, glycoproteins, lipoproteins, and enzymes.
Low albumin can result in ascites, and low prothrombin, fibrinogen, and other clotting
factors can lead to a hypocoagulable state. Hypoglycemia can result from failure of
the damaged hepatocytes in maintaining glucose homeostasis (4). A major function
of the hepatocyte is the conjugation of bilirubin and its excretion into the bile
canaliculi. A sign of hepatic injury is not elevated unconjugated bilirubin, but rather
conjugated hyperbilirubinemia, which may be due to the decreased excretion of
conjugated bilirubin (cholestasis) due to inflammation around the canaliculi. The
build up of bilirubin in the bloodstream leads to jaundice or icterus, which is a yellow
coloring of the skin and sclera (of note, scleral icterus can be seen when the bilirubin
level exceeds 2.5 mg/dl) (5). Note that only some patients with hepatitis are
jaundiced because only some patients develop cholestasis. Ammonia is a normal
byproduct of protein degradation by intestinal bacteria, of deamination processes in
the liver, and glutamine hydrolysis in the kidneys. The liver metabolizes this toxic
ammonia into urea by the Krebs-Henseleit cycle. With hepatic injury, however, the
ammonia may accumulate which can lead to encephalopathy and coma (6).
With this short explanation on the pathophysiology of hepatic parenchymal injury, the
diseases that cause hepatitis can be more readily understood. This next section will
focus primarily on viral hepatitis, in addition to two major causes of metabolic
diseases of the liver: Wilson disease and alpha-1-antitrypsin deficiency.
The viral causes of hepatitis can be divided into hepatotropic and non-hepatotropic
viruses. The non-hepatotropic viruses include measles, rubella, enteroviruses
(coxsackie and echo), flaviviruses (yellow fever, Dengue fever), filoviruses (Marburg
and Ebola), arenaviruses (Lassa fever), parvovirus B19, adenovirus, and herpesviruses
(herpes simplex types 1 and 2, varicella-zoster virus, cytomegalovirus, Epstein-Barr
virus, and human herpes virus type 6) (7). The hepatotropic viruses are fewer in
number and consist of hepatitis A, B, C, D, E, and G. The hepatitis viruses important
in clinical medicine are hepatitis A, B, C, and delta.
Hepatitis A (HAV) is a picornavirus (single-stranded RNA virus) and is transmitted
via the fecal-oral route. After it is ingested, it replicates in the small intestine, and
then travels to the liver via the portal vein, where it attaches to hepatocytes via a
receptor on the hepatocyte membrane. The replicated HAV is then excreted in the
bile and passed on in the feces. Liver injury is thought to be due to a T-cell mediated
destruction of hepatocytes, rather than to a direct cytotoxic effect. Important risk
factors for acquiring HAV infection are close contact (including sexual), fecal-oral
(i.e., poor hand washing, etc.), travel to developing countries, and intravenous drug
abuse. Therefore, parenteral transmission of HAV is possible, although uncommon.
The incubation period of HAV is 15 to 40 days, with a mean of 28 days, after which
the patient can develop clinical manifestations of hepatitis. In older children and
adults, symptoms can include fever, anorexia, nausea and vomiting, right upper
quadrant abdominal pain, dark urine, and jaundice. Less common symptoms include
headache, myalgias, arthralgias, pruritus, and rash.
Signs can include
hepatosplenomegaly, leukopenia, cervical lymphadenopathy, hyperbilirubinemia, and
elevation of serum aminotransferase levels. Fulminant hepatic failure is rare. In
infants and younger children, HAV can be asymptomatic or present with "stomach-flu
symptoms" of nausea, vomiting, and diarrhea without icterus; therefore suspicion of
hepatitis in these patients is often low. Diagnosis is made by measuring anti-IgG and
anti-IgM HAV titers. Anti-IgM is present in the acute period, peaking at 1 week and
disappearing 3 to 6 months later. Anti-IgG appears later and is highest at 1 to 2
months and lasts for years, and therefore signifies convalescence or immunity. The
treatment for HAV is mainly supportive, and the prognosis is good in that it is acute
and self-limiting. It does not lead to chronic hepatitis or chronic carriage. In a small
percentage of patients, HAV infection can be relapsing or protracted. Prevention
consists of good hygiene, the administration of immunoglobulin prophylaxis to
contacts, and active vaccination (8,9). Intramuscular immunoglobulin when given as
postexposure prophylaxis has >85% effectiveness in preventing symptomatic
infection if given in the first 2 weeks of exposure; however, its immunity is shortlived. Active HAV vaccination is recommended for preexposure prophylaxis to
people traveling to foreign countries with a high rate of HAV (excludes Australia,
Canada, Japan, New Zealand, Western Europe, and Scandinavia).
Other
recommendations for vaccination are children living in areas of the United States with
an incidence rate twice the national average (which includes Arizona, Alaska, Oregon,
New Mexico, Utah, Washington, Oklahoma, South Dakota, Idaho, Nevada, and
California), people with chronic liver disease, homosexual and bisexual men, IV drug
users, patients with clotting-factor disorders, and those who are at risk for
occupational exposure) (10).
Hepatitis B (HBV) is a DNA virus in the family hepadnaviridae and has a unique
structure. It consists of two shells, of which the outer shell contains the hepatitis B
surface antigen (HBsAg) and the inner shell contains the hepatitis B core antigen
(HBcAg). In the core are the viral DNA and the hepatitis B e antigen (HBeAg) which
is a derivative of the precore DNA region of the HBcAg gene. Like the hepatitis A
virus, it has a high affinity for hepatocytes and causes destruction through T-cell
mediated cell destruction. HBV is transmitted parenterally and is found in all bodily
fluids, but is not found in feces. Although it is found in saliva, the chances of
acquiring HBV through sharing of toys by young children are small. Sexual
intercourse and vertical transmission (from mother to infant) are other important
routes of transmission. Vertical transmission, which is a major cause of acquiring
HBV in endemic countries, can occur through the infant swallowing maternal blood
during birth or transplacentally. In addition, infants who acquire HBV perinatally
have a high risk for chronic hepatitis infection, cirrhosis, and hepatocellular
carcinoma. The incubation period is from 50 to 180 days. The clinical course has
three stages: prodromal (incubation) stage, symptomatic (icteric) stage, and
convalescent stage. The prodromal stage lasts for 2 to 3 weeks and although in most
cases the patient is asymptomatic, it can rarely present with membranous nephropathy
and vasculitic syndromes. This is followed by the symptomatic (icteric) stage that
consists of fatigue, fever, myalgias, anorexia, pruritus, nausea, vomiting, and
abdominal pain. This stage can last for 4 to 6 weeks. An interesting dermatologic
phenomenon that can occur is papular acrodermatitis of childhood (Gianotti-Crosti
syndrome), which presents as nonpruritic symmetrical lichenoid papules on the face,
buttocks, and extremities that last for 2 to 3 weeks and often preceded by
lymphadenopathy, hepatomegaly, and occasionally splenomegaly (11).
The
extrahepatic manifestations are possibly due to immune complexes of HBsAg and
anti-HBs. After this stage is the convalescent stage (8).
The diagnosis of HBV is made by interpretation of serological markers of HBsAg,
HBeAg, anti-HBe, anti-HBc, and anti-HBs, (note the absence of HBcAg which can
only be assayed on a liver biopsy, since it does not circulate in the blood in
appreciable quantities) and the patient's clinical state. It would be helpful to look at a
graph of these markers and course of disease for a visual depiction. However, these
graphs are confusing to most students so it would be better to understand where these
markers are from and their immunologic/clinical consequence. After inoculation,
viral replication occurs. So during incubation and replication of the virus, one would
find viral antigens in the serum, namely HBsAg and HBeAg. In fact, these antigens
are not found in the blood until 1 to 3 months after the person becomes infected.
Patients with these circulating antigens are potentially contagious. A positive HBsAg
together with a positive HBeAg is associated with a higher degree of contagiousness
than a positive HBsAg alone. While the patient has these antigens then, he is infected
and is either in the incubation or symptomatic stage of the disease (acute or chronic).
In the meantime, the immune system is fighting off this infection with the production
of antibodies against these antigens. For example, Anti-HBs is made in response to
HBsAg. The antibody and antigen should not be detectable in the blood stream
simultaneously.
Basically, the presence of HBsAg indicates infection and
contagiousness, while the presence of anti-HBs indicates the presence of immunity.
However, there is a brief period after the HBsAg declines and before the anti-HBs
rises, when both of them are non-detectable (negative), which is called the "window
phase". Anti-HBc is always positive during this window phase.
In acute HBV infection, HBsAg and HBeAg are present. If recovery and immunity
are to follow, both antigen levels decline as anti-HBc and anti-HBe levels rise.
Ultimately, the presence of anti-HBs signifies immunity. In chronic HBV infection,
HBsAg persists and anti-HBs fails to develop. Once anti-HBs and/or anti-HBc are
present, these can be fractionated to IgM and IgG. IgM signifies an early immune
response, while IgG signifies a later or booster response. It may be best to understand
these antigens and antibodies in relation to specific clinical questions.
Is the patient immune or contagious? If the HBsAg is positive, the patient is
contagious. If the HBsAb is positive, the patient is immune.
Is a pregnant mother at risk for passing HBV to her child at birth? If mother's HBsAg
is positive, then perinatal transmission of HBV is possible and the newborn must
receive HBV prophylaxis. If the HBsAg is negative, there is no risk.
The patient is contagious (i.e., the HBsAg is positive), but how contagious is he? If
the HBeAg is positive, he is very contagious. If the anti-HBe is positive, then he is
less contagious.
Has the patient been exposed to HBV in the past? Check the HBsAg, anti-HBc and
anti-HBs. If all of these are negative, the patient has never been exposed to HBV. If
one or more are positive, then the patient has been exposed to HBV.
A hepatitis B vaccine recipient wants to confirm immunity. If the anti-HBs is
positive, he is immune. If not, immunity has not yet developed.
The prognosis of HBV infection is variable and depends on immune and genetic
factors, the age of the patient, and serologic stage of infection. The risk for chronicity
increases when primary infection is acquired in the neonatal period compared to
adults. For instance, 95% of neonates become chronic carriers, compared to 20% of
children, and less than 10% of adults. Chronic states can range from chronic active
hepatitis to a chronic non-symptomatic carrier state. Also, the risk for hepatocellular
carcinoma (HCC) increases with chronicity; therefore, patients who develop HBV in
the neonatal period have the highest risk of developing liver cancer compared to other
age groups. It has been shown that men who develop HBV at birth have a 50%
lifetime risk for developing HCC compared to women who have a 20% lifetime risk.
Patients who develop HCC have a poor prognosis, which has a less than 5% 5-year
survival rate (8).
Fortunately, the risk of HBV has declined with universal immunization and diligent
prenatal screening. Infants who are born to HBsAg-negative women are given the
routine 3-dose hepatitis B immunization, with preterm infants given the first dose
after they reach 2 kg. However, newborns from HBsAg-positive women are given
both the hepatitis B vaccine and hepatitis B immunoglobulin (HBIG) as postexposure
prophylaxis within 12 hours of birth regardless of their birth weight. For infants less
than 2 kg, the initial dose should not be counted in the 3-dose schedule (i.e., they are
given 4 doses). After completion of the HBV vaccine series, these infants born to
HBsAg positive mothers should be tested serologically for anti-HBs and HBsAg 1-3
months after the last dose. For infants who have low anti-HBs (<10 mIU/ml) and
negative HBsAg, they should receive 3 additional doses of vaccine at a 0, 1, and 6month schedule, with anti-HBs testing done 1 month later to determine immunity. If
the HBsAg status of the mother is unknown, serologic testing on the mother should be
performed as soon as possible. Term infants should receive the hepatitis B vaccine
within 12 hours of birth. HBIG does not need to be given unless the mother's
serology returns as being HBsAg positive, in which case HBIG is given soon after.
Two hepatitis B vaccines are currently available (Recombivax and Engerix). The
schedule consists of 3 doses, with the first dose being given soon after birth, a second
dose at least 1 month later, and the third dose when the infant is at least 6 months old
or at least 2 months after the second dose and at least 4 months after the first dose
(12,13). HBV vaccine is also available in a combination vaccine with diphtheriatetanus-pertussis (DTaP) and inactivated polio vaccine (IPV).
Hepatitis C was a major cause of post-transfusion hepatitis in the past and was known
as non-A, non-B hepatitis prior to the late 1980's. It belongs to the family
flaviviridae, which are enveloped RNA viruses. It is spread parentally with highest
risk factors being illicit intravenous drug use and unprotected sexual intercourse.
Although it is an important cause of posttransfusion hepatitis, only 5 to 10% of HCV
infections are due to transfusions. Another risk factor is needlestick injuries in
hospital workers. Vertical (perinatal) transmission is probably the most common
cause of HCV in children, although overall, it is uncommon for an infant to acquire
HCV compared to HBV. HCV is thought to have a direct cytotoxic effect, which is in
contrast to HAV and HBV. Clinical manifestations are the same for the other forms
of hepatitis B, with most cases being anicteric. However, unique features are
fluctuating or polyphasic levels of aminotransferases during the course of disease and
slow resolution. In 50% of individuals, chronic HCV infection can occur. Out of this
50%, half become chronic carriers and the other half progress to chronic active
infection or chronic persistent infection. For those who are chronic carriers, they are
asymptomatic although test positive for HCV. For those who develop chronic
infection, 20% will develop cirrhosis and are at high risk for hepatocellular
carcinoma. There is no vaccine available against HCV due to its high rates of
mutation in its viral envelope, although children who do develop HCV infection have
been treated successfully with interferon alpha (8,14).
Hepatitis D virus (HDV) or delta agent is a satellite virus, meaning that it can only
cause disease, not by itself, but in conjunction with another virus, which in this case is
hepatitis B virus. HDV is a very small RNA virus and is thought to damage
hepatocytes by a direct cytotoxic effect. Like HBV, it is spread parenterally.
Unfortunately, those patients with HBV who are co-infected with HDV have severe
forms of hepatitis. Mortality is higher at 2% to 20% compared to less than 1% for
those with only HBV infection. Most patients (about three-quarters) with chronic
HDV infection develop cirrhosis and portal hypertension (8).
Two forms of metabolic liver diseases will be discussed next: Wilson disease and
alpha-1-antitrypsin deficiency. Wilson disease or hepatolenticular degeneration is a
disease of copper metabolism. It is autosomal recessive with the affected gene,
ATP7B, being on the long arm of chromosome 13. It occurs worldwide, with a
prevalence of about 1 in 30,000, with higher rates in consanguineous families.
Mutations in this gene cause impaired copper excretion from the hepatocyte to bile,
and decreased incorporation of copper into ceruloplasmin in the hepatocyte leading to
high serum copper levels and deposition of copper in many organs. The clinical
manifestations of Wilson disease rarely appear until 5 years of age, at which time the
gradual build up of copper in various organs becomes symptomatic. Dangerous levels
of copper become present in the liver, nervous system, cornea, kidneys, and other
organs leading to hepatitis, neuropsychiatric symptoms (some of which are tremor,
clumsiness, ataxia, headaches, seizures, and dementia), and Kayser-Fleischer rings
around the corneas (a greenish-brown ring in Descemet's membrane at the periphery
of the cornea). The diagnosis is made on the basis of several tests and clinical data.
Serum ceruloplasmin is low (<20 mg/dl), hepatic copper concentration is high (>250
mcg/gm of dry wt.), 24-hour urine copper excretion is high (>100 mcg/24 hours),
Kayser-Fleischer rings are present, and incorporation of 64Cu into ceruloplasmin is
low. Without treatment, Wilson disease is fatal; therefore, the basis of therapy targets
the reduction of stored copper and preventing reaccumulation of copper. This is done
by using copper-chelating agents (e.g., D-penicillamine, trientine, zinc acetate,
ammonium tetrathiomolybdate, and vitamin D), a low copper diet, oral zinc therapy,
and possibly antioxidants (15).
Alpha-1-antitrypsin deficiency (A1AT deficiency) is another important metabolic
liver disease that is autosomal recessive and occurs in 1 in 1,600 to 2,000 live births.
Alpha-1-antitrypsin is a glycoprotein that inhibits neutrophil proteases such as
neutrophil elastase, cathepsin G, and proteinase 3. The absence of alpha-1-antitrypsin
allows these dangerous enzymes to cause damage to organs. The two organs that are
affected are the liver and lung. Hepatic manifestations include prolonged jaundice in
infants; neonatal hepatitis syndrome, and mild elevations of aminotransferases in
toddlers; portal hypertension and severe liver dysfunction in older children; chronic
hepatitis, cryptogenic cirrhosis, and hepatocellular carcinoma in adults. The
pulmonary manifestation is emphysema, although this commonly occurs in adult
cigarette smokers and rarely in children. A1AT deficiency is suspected in infants
with neonatal jaundice or older children and adults with unexplained chronic liver
disease. The diagnosis is made by determining the phenotype of serum alpha-1antitrypsin by electrophoresis or isoelectric focusing, and confirmation by liver
biopsy. The phenotype associated with liver disease is an individual homozygous for
PiZZ (ZZ phenotype of the protease inhibitor system). The treatment of liver disease
is by liver transplantation, and emphysema with lung transplantation and cessation of
cigarette smoking. Gene therapy may be possible in the future (16, 17).
Lastly, the work-up of hepatitis should be done in a systematic and stepwise fashion.
Initially, a complete blood count, total and fractionated serum bilirubins, aspartate
aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl
transferase (GGT), and prothrombin time (PT) should be performed. AST, ALT, and
GGT assess for hepatocellular damage (one could argue that only one of these is
necessary) and PT is the most useful test to determine if liver dysfunction is present.
If these tests are abnormal and the etiology is unknown, viral hepatitis titers consisting
of anti-HAV IgM, HBsAg, anti-HBc, and anti-HCV should be performed. If these
tests are negative, other viruses such as Epstein-Barr virus and cytomegalovirus
should be considered. If these tests are still negative, other etiologies should be
sought after. These etiologies include biliary atresia in neonates (refer to the chapter
on biliary atresia), Wilson disease by ceruloplasmin and 24-hour urinary copper
excretion, cystic fibrosis by sweat chloride, tyrosinemia by urinary succinyl acetone,
alpha-1-antitrypsin deficiency by serum alpha-1-antitrypsin and protease inhibitor
phenotyping, and autoimmune hepatitis by presence of autoantibodies and
hypergammaglobulinemia (18).
In summary, although viral hepatitis can be self-limited, hepatitis B, C, and delta can
cause cirrhosis, death, and liver cancer. Despite the nature of these infections,
excellent vaccines can prevent the most common one, hepatitis B. In fact, the
hepatitis B vaccine is unique in that it is the only vaccine that can prevent cancer.
With the advent of new technologies and gene therapies on the horizon, the outlook
for liver disease is favorable.
Questions
1. Why are aminotransferases, alkaline phosphatase, and GGT not considered
liver function tests? What is the most useful test for liver function?
2. True/False: Most infants and young children with hepatitis A present with
jaundice.
3. A family is planning a vacation in China that is known to have a high rate
of hepatitis A. How would you give preexposure prophylaxis to this family who has a
15 month old and a 5 year old child?
4. A labor and delivery nurse informs you that a term infant was just born
whose mother is HBsAg negative and anti-HBs positive. How would you approach
this infant for prophylaxis?
5. A 1600 gm infant is born to a mother who is HBsAg positive, anti-HBc
positive, and anti-HBs negative. The NICU nurse is asking what your order is for this
patient. Out of these three HepB tests, which one is the most useful in your decision
making process.
6.
Name three organ systems involved in Wilson disease and its
manifestations.
7. What element is implicated in Wilson disease?
8. What organ systems are involved in alpha-1-antitrypsin deficiency and
what are their manifestations?
References
1. Davis FA. Taber's Cyclopedic Dictionary, 16th edition. 1985,
Philadelphia: F.A. Davis Company, pp. 450, 1044.
2. Shneider BL, Suchy FJ. Chapter 361-Autoimmune Hepatitis. In:
Behrman RE, et al (eds). Nelson Textbook of Pediatrics, 16th edition. 2000,
Philadelphia: W.B. Saunders Company, pp. 1216-1218.
3. Kelly DA. Chapter 1-Useful Investigations in the Assessment of Liver
Disease. In: Kelly DA. Diseases of the Liver and Biliary System in Children. 1999,
Massachusetts: Blackwell Science, Ltd., pp. 3-10.
4. Colon AR. Chapter 2-General Considerations. In: Colon AR (ed).
Textbook of Pediatric Hepatology, 2nd edition. 1990, Massachusetts: Year Book
Medical Publishers, Inc., pp. 6-15.
5. Colon AR. Chapter 3-Signs and Symptoms. In: Colon AR (ed).
Textbook of Pediatric Hepatology, 2nd edition. 1990, Massachusetts: Year Book
Medical Publishers, Inc., pp. 16-29.
6. Colon AR. Chapter 4-Diagnostic Aids. In: Colon AR (ed). Textbook of
Pediatric Hepatology, 2nd edition. 1990, Massachusetts: Year Book Medical
Publishers, Inc., pp. 30-47.
7. Davison S. Chapter 4-Acute Hepatitis. In: Kelly DA (ed). Diseases of the
Liver and Biliary System in Children. 1999, Massachusetts: Blackwell Science, Ltd.,
pp. 65-76.
8. Yazigi NA, Balistreri WF. Chapter 17-Acute and Chronic Viral Hepatitis.
In: Suchy FJ, Sokol RJ, Balistreri WF (eds). Liver Disease in Children, 2nd edition.
2001, Philadelphia: Lippincott Williams & Wilkins, pp. 365-428.
9. Tanuos H. Hepatitis A. Pediatr Rev 1999;20 (3):102.
10. American Academy of Pediatrics. Hepatitis A. In: Red Book 2000, 25th
edition. 2000, Illinois: American Academy of Pediatrics, pp. 280-289.
11. Hurwitz S. Chapter 5-Papulosquamous and Related Disorders. In:
Hurwitz S. Clinical Pediatric Dermatology, 2nd edition. 1993, Philadelphia: W.B.
Saunders Company, pp. 105-135.
12. American Academy of Pediatrics. Hepatitis B. In: Red Book 2000, 25th
edition. 2000, Illinois: American Academy of Pediatrics, pp. 289-302.
13. Centers for Disease Control and Prevention. Chapter 14-Hepatitis B. In:
Atkinson W, Wolfe C, Huminston S, Nelson R (eds). Epidemiology and Prevention
of Vaccine-Preventable Diseases, 6th edition. 2000, Atlanta: Public Health
Foundation, pp. 207-229.
14. Rajan-Mohandas N. Hepatitis C. Pediatr Rev 1999;20(9):323.
15. Sokol RJ, Narkewicz MR. Chapter 26-Copper and Iron Storage
Disorders. In: Suchy FJ, Sokol RJ, Balistreri WF. Liver Disease in Children, 2nd
edition. 2001, Philadelphia: Lippincott Williams & Wilkins, pp. 595-640.
16. Perlmutter DH. Chapter 23-Alpha-1-Antitrypsin Deficiency. In: Suchy
FJ, Sokol RJ, Balistreri WF. Liver Disease in Children, 2nd edition. 2001,
Philadelphia: Lippincott Williams & Wilkins, pp. 523-548.
17. Balistreri WF. Chapter 357-Metabolic Diseases of the Liver. In:
Behrman, et al (eds). Nelson Textbook of Pediatrics, 16th edition. 2000,
Philadelphia: W.B. Saunders Company, pp. 1207-1212.
18. Rosenthal P, Lightdale JR. Laboratory Evaluation of Hepatitis. Pediatr
Rev 2000;21(5):178.
Answers to questions
1. These enzymes are found within the hepatocyte, and therefore are
indicative of hepatocellular damage, and not actual function of the liver. The most
useful test for liver function is prothrombin time.
2. False. They are usually anicteric.
3. The 15 month old should receive immunoglobulin (too young to receive
Hep A vaccine). The 5 year old can receive the Hep A vaccine since she is over 2
years of age. The vaccine is given as two doses 6 months apart.
4. The mother is actually immune to hepatitis B, perhaps from receiving
hepatitis B vaccinations in the past or from a previous exposure to hepatitis B. She
does not have infection, she is not contagious and in fact, she is immune. This infant
does not need HBIG prophylaxis, but should be vaccinated against hepatitis B in the
usual fashion.
5. The mother has a positive HBsAg, which means that she is contagious.
Therefore, this infant needs HBIG and hepatitis B vaccine prior to 12 hours of age.
Because this premie is less than 2 kg, a 3-dose vaccine schedule should be instituted
after this infant is over 2 kg, and not counting the initial dose because he was less than
2 kg. After completion of the 3-dose schedule, he should be tested serologically for
anti-HBs and HBsAg 1-3 months after completion of the series. If his anti-HBs (<10
mIU/ml) is low and HBsAg is negative, then he should receive 3 additional doses of
vaccine at a 0, 1, and 6-month schedule, with anti-HBs testing done 1 month later to
determine immunity. The mother's status could be consistent with acute hepatitis B,
chronic hepatitis B, or a hepatitis B carrier state. The most important serologic test
out of the three listed is the HBsAg, since this test tells us whether the mother is
contagious and the newborn requires HBIG prophylaxis.
6.
Brain (or nervous system), liver, and eye.
Manifestations are
neuropsychiatric symptoms, hepatitis, and Kayser-Fleischer rings.
7. Copper.
8. Lung and liver. The pulmonary manifestation is emphysema and hepatic
manifestations include prolonged jaundice in infants, neonatal hepatitis syndrome,
mild elevations of aminotransferases in toddlers, portal hypertension and severe liver
dysfunction in older children, and chronic hepatitis, cryptogenic cirrhosis, and
hepatocellular carcinoma in adults.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
Gastro esophageal reflux:
A one month old male is brought to your office by his first time parents with a
complaint of constant irritability and spitting up. The 2.8 kg (6 pounds, 3 ounce)
product of an uneventful full term pregnancy and delivery, he was discharged on the
second day of life. He always seems to be hungry, and since his mother is certain that
she is not producing enough milk, she has been following the breast feedings with
formula for the last 2 weeks. He currently will feed at the breast for 10 minutes, then
consume another 4 ounces by bottle. When left with his grandparents, he will finish
an entire 8 ounce bottle in 5-10 minutes and they report he will cry if they try to cut
him off at the recommended 4-5 ounces. The vomiting generally occurs immediately
after feedings. It is not forceful, nor is it blood or bile-tinged. He fills 10 diapers with
urine daily, and lately he has been having watery stools, which have further worried
his grandparents. Despite all this, he weighed 3.5 kg (7 pounds, 11 ounces) at the two
week checkup and he now weighs 4.3 kg (9 pounds, 8 ounces).
Exam: VS are unremarkable. His physical examination is notable only for fussiness
when laid supine on the table, with resolution when held upright or in the prone
position. You witness effortless regurgitation of 2-5 ml of curdled formula every few
minutes during the history and exam since his parents "topped him off" with formula
in your waiting room before the appointment as he was beginning to fuss.
Gastroesophageal (GE) reflux is defined by the North American Society for Pediatric
Gastroenterology and Nutrition (NASPGN) as the "passage of gastric contents into
the esophagus" (1). This is a normal physiologic process including regurgitation (the
generally low pressure passage of gastric contents up to the mouth) as opposed to
vomiting (the forceful expulsion of gastric contents via the mouth) as the latter is
more often associated with obstruction or other significant abnormal alteration of
gastric motility involving reversal of the usual gastric emptying phenomenon.
Likewise, it is to be differentiated from rumination, which is the purposeful return of
gastric contents to the mouth as a response to behavioral issues, most typically
beginning in the second half of the first year of life and occurring in neglected infants
and children in part as self-stimulatory behavior or as a means of getting attention
from an otherwise markedly non-interactive (and usually clinically depressed)
caretaker.
GE reflux occurs at all ages, and its consideration is best divided between infantile
reflux and reflux in the older toddler/child, as the presentations will differ due to
responses available due to developmental stage. It can be a chronic and a recurrent
problem.
In infants, the more typical presentation is as above, which the NASPGN label "the
happy spitter" (1) who freely regurgitates, but more commonly than not, has no sign
of respiratory compromise. With the relatively low acid secretory capability and the
constant feeding of early infancy, there is less tendency to irritability suggestive of
dyspepsia, though many (like the child in the example) will show some sign, and
some will become markedly colicky. The attribution of the colicky behavior to reflux
is supported by an increase in fussiness in positions where reflux would be promoted;
such as supine or slumped in a mal-positioned baby seat, or at times when reflux can
be expected; such as following an overfeeding as in our example.
In toddlers and older children, overt regurgitation is less common as they spend more
time upright and typically will have learned eating behaviors favoring solids and
minimizing liquids which further help retain most of the feedings in the stomach. The
retention is not complete, however, and they more typically present with symptoms or
signs suggestive of distal esophageal irritation. Aside from complaints of epigastric
pain (in the pre-verbal toddler often indicated as holding the epigastrium or refusing
to eat further), they can include drooling (caused by reflex hypersalivation triggered
by the acid sensors of the distal esophagus acting via the brainstem on the salivary
glands), or pronounced eructation (i.e., belching, representing regurgitation of air coswallowed with the saliva). The latter two are manifestations of the esophageal
protective mechanisms, and can be seen in early infancy presentations, just as many
toddlers will still regurgitate freely. In the older child and adolescent, hypersalivation
is more commonly manifest as a sleeping behavior (as not all the saliva produced
while recumbent is swallowed) and often is accompanied by sleep in specific
positions of comfort, the most common of which are prone and left decubitus as these
offer some positional advantage to mitigate reflux. These options are not available to
the infant (particularly in the face of the American Academy of Pediatrics' "Back to
Sleep" campaign against SIDS), often resulting in worsening colicky behavior, as
noted in our example.
Occasional patients will present with respiratory symptoms as their primary complaint
with reflux laryngitis and the contribution of microaspiration of either regurgitated
acid or oral secretions (from the hypersalivation) in the exacerbation of chronic
asthma is gaining increasing recognition. Though more common as a presenting
complaint among older children, it will occur in younger children as well, but is not
the more common presentation for any age. A more worrisome presentation is frank
aspiration or choking, resulting in pneumonia for the former and symptoms ranging
from gagging and sleep interruption to apparent life threatening events (ALTE) for the
latter. These more serious conditions require full regurgitation, and are also far less
common than the non-respiratory symptoms which require reflux only part-way up
the esophagus. The NASPGN have acquiesced to the AAP in dissuading prone
sleeping position before 12 months of age, though proning may be used while "awake,
particularly in the postprandial period" (1).
GE reflux, and more specifically secondary esophageal irritation (if not frank
esophagitis), can result in voluntary reduction in intake in all ages, resulting in classic
failure to thrive or frank weight loss. It can result in overt feeding refusal, though it
more commonly is manifested as a selective intake, avoiding items which cause pain
including acidic and spicy foods, and surprisingly commonly, items with adverse
effect on the distal esophagus, including caffeine and chocolate if the examiner
questions specifically.
GE reflux must be differentiated from vomiting, as the latter hints at obstruction, and
can also arise from metabolic processes (urea cycle defects and Reye syndrome) as
well as disease in other organ systems (increased intracranial pressure, pancreatitis,
urinary tract infection, or distention of any hollow viscus such as the gallbladder or
renal pelvis/ureters). It should also be differentiated from extra-abdominal causes
such as post-tussive vomiting, or altered motility due to allergic enteritis or
eosinophilic gastroenteritis. In the case above, a one month old with projectile
vomiting would suggest pyloric stenosis, but in our case the vomitus is not forceful
and has been present from the neonatal period. Projectile vomiting requires an intact
lower esophageal sphincter (LES) function to develop adequate intragastric pressure.
Since GE reflux is common at this age (>50%), projectile vomiting may not be
present in infants with coexisting pyloric stenosis with GE reflux, which may delay
the diagnosis of pyloric stenosis.
Three mechanisms produce the majority of GE reflux: 1) Chronically decreased
lower esophageal sphincter tone is most common at all ages and can be expected to
improve over the first year of life. It is characterized by symptoms which occur more
commonly immediately after feedings and further reflect effects of posture or intraabdominal pressure. 2) Delayed gastric emptying is common among adults due to
progressive gastroparesis, particularly with diabetes mellitus, but is less common in
children. Characteristically it will produce symptoms which continue for hours after
feedings, reflecting the persistently full stomach. 3) Inappropriate LES relaxation is
least common of the three, and typically produces more irregular timing of the
symptoms, which tend to be more fleeting as the esophageal protective mechanisms
are typically more effective in these youngsters than with the other two.
The diagnosis of GE reflux is typically made by a detailed history and physical
examination alone. Regurgitation reliably reported or observed with appropriate
adjunct symptoms and signs is suggestive of uncomplicated GE reflux. A careful
elucidation of a consistent constellation of symptoms can suggest reflux which is not
visible (which is also sufficient to trigger the first lines of intervention). It is in
situations where significant secondary disease is present (such as recurrent aspiration,
stridor suggesting laryngeal irritation, or failure to thrive with or without frank
feeding refusal), that subspecialist assistance should be sought at an early stage, even
if overt regurgitation makes the diagnosis fairly certain. Efforts should be made to
exclude the other items in the differential diagnosis above, but many can be excluded
on the basis of a good history and physical examination of the relevant organ systems.
Radiographic studies are not part of the usual initial workup since the absence of
reflux on a short radiographic study cannot rule out GE reflux, and the manipulation
inherent in the exam can itself trigger regurgitation. The main utility of the upper
gastrointestinal contrast study is to search for structural anomalies such as malrotation
as well as the much rarer webs and secondary strictures. These are often
accompanied by signs of obstruction (though bilious vomiting may be absent if the
obstruction is proximal to the mid-duodenum). The exception is the younger patient
with signs of tracheomalacia, as the rare vascular ring, trapping both the esophagus
and trachea in its grasp during in utero growth, deserves early intervention. Another
exception is pyloric stenosis, for which ultrasound provides less invasive evaluation,
permitting earlier access to surgery.
The radionuclide gastric emptying study, likewise is not commonly part of an initial
workup, as its prime utility is in assessing delayed gastric emptying. Unfortunately,
age appropriate standards are not well established, prompting the use of this test in the
more severe cases where surgery is already being contemplated (typically
fundoplication). Scintigraphic imaging during the hour-long study can also identify
reflux visually (but again cannot rule it out due to the short duration of the study) and
24 hour delayed imaging is cited as being of utility in searching for evidence of
aspiration.
pH probes offer a means of assessing the frequency and duration of acid reflux, and
with the double-sensor probes, the differentiation between regurgitation and reflux
only part-way up the esophagus can be made. Twenty-four hour studies are more
reliable than those of shorter duration, since reflux varies with activity and sleep state.
Their prime utility is in the patient with symptoms which are clear and disruptive who
does not have a clear association with visible regurgitation. The classic example is
the infant with repeated ALTEs who is found with curdled feedings. The main issue
in such patients in establishing causality is determining whether the reflux came first,
then the obstruction, then the apnea. It is more common that the apnea came first,
then the agonal relaxation of the LES and regurgitation. In such situations,
simultaneous multichannel recordings are essential, since the transition from one
stage to the next may be only seconds apart, and on such studies many infants with
obstructive apneic episodes and known GE reflux have the two occur at completely
different times.
Manometric studies have fallen on disfavor, as they do not address the issues of
delayed gastric emptying or inappropriate relaxation of the LES.
For the average healthy infant with no threatening complications, the GE reflux can
be approached first with basic mechanical measures:
1) Regulate feedings: As in our illustration, overfilling of the stomach is to be
discouraged, and in such a patient, I would recommend the bottle feedings be halted if
there is ample evidence of sufficiency of breast feedings. This can be reinforced by
following the urine output, with most parents being reassured when told that the fluid
urinated had to have been absorbed, and the nutrients associated with that fluid can be
expected to be absorbed as well. In the bottle-fed infant, the volume can be
calculated, but I have found it easier to give the caretakers a means of identifying the
volume that would fit in a minimally distended stomach as being roughly a quarter of
the abdominal volume as measured between the ribs and the pelvic brim. This
forestalls repeated questions of "how much can we feed now?" as the child grows.
The feedings also need to be regularly spaced, to avoid overfilling with too closely
spaced feedings. This is less of a problem in the exclusively breast-fed infant, but is
not eliminated. For the demanding infant, use of suitable pacification (particularly a
parental digit) can be helpful. The feedings also need to be evenly paced, to allow
enough time for the infant to feel full and cut off the feeding before overfilling occurs.
With the bottle-fed infant, thickening of the feedings is possible; in exclusive breastfeeding, the parental digit will again have to be used.
2) Positioning: Maximizing the vertical distance between the mouth (or more
particularly the larynx) and the stomach helps minimize reflux and secondary
esophageal irritation (i.e., colic). The literature does support prone positioning in this
regard, but the cautions noted above regarding SIDS apply. It is worth mentioning to
parents, however that infants choose their own sleeping positions once they are able to
roll from supine to prone around 4 months of age to avoid many sleepless nights
repeatedly rolling their infant back into the supine position only to flip back as soon
as he or she is free to do so. Decubitus positioning provides some relief, as can
positioning in a recliner (as long as the angle chosen does not cause slumping). There
will be times when carrying the infant upright may offer the only relief (particularly
after overfeeding).
3) Thickening the feedings may be a consideration in the formula fed infant,
and is far less practical in the breast-fed one. In many cases the greater utility of the
thickening is in slowing the feeding rate than in any retention within the stomach.
Rice cereal is preferred over the recently introduced formulas that thicken when
exposed to acid (recall many young infants may not produce much acid). Typical
recipes call for one-half to one tablespoon of rice cereal per ounce of formula, which
also adds substantially to the overall caloric intake. Thickening to encourage
retention in the stomach is of most use in those with evidence of chronic low LES
tone (spitting which occurs predominantly shortly after the meal) and can be less than
useful in those who have delayed gastric emptying (with spitting which continues for
hours after a meal) as it may cause further delay in emptying. In such infants who are
formula fed, one of the cheaper partially hydrolyzed formulas may provide the better
option, as fluids empty from the stomach faster than curd. In that respect, breast
feeding, with its thinner curd, tends to empty faster than most formulas.
In older toddlers and children:
1) Regulate the feedings: Many with secondary esophageal irritation (if not
frank esophagitis) will tend to complain of nausea and anorexia in the morning, and
skip or minimize breakfast intake. They may or may not eat much lunch, particularly
if the school is providing a spicy menu. They often eat more of their daily caloric
intake throughout the afternoon and evening. Redistributing the intake to be more
evenly spaced during the day will result in less nocturnal acid reflux and is of most
utility in those complaining of symptoms after supper or nocturnal waking or morning
nausea. Avoidance of after supper snacking can also help.
2) Positioning is less of a problem once infants pass 6 months of age and can
choose to be upright. For older children, the option of elevation of the head of the bed
for sleep is often declined as more seem to prefer prone positioning.
3) Avoid agents prone to adversely altering LES tone and functioning such as
caffeine and nicotine.
In all age groups, a therapeutic trial to address acid can be of significant
diagnostic utility. My personal preference is to use antacids, since this provides
immediate pain relief (good reinforcement). Typical therapeutic courses with
histamine-2 receptor blockers or proton pump inhibitors run 6-8 weeks with only
partial resolution. In infants, the aluminum containing antacids should be avoided
since aluminum absorption may cause osteodystrophy. A typical therapeutic trial
yields suggestive results within 2 weeks, and can be helpful in determining whether
an atypical (but non-threatening) symptom is acid-related.
Beyond these basic steps, the evaluation and therapy diverge based on the dominant
symptoms. If delayed gastric emptying is the issue, therapy centers on properistaltic
agents and may include a more thorough evaluation of structure and gastric emptying.
If pain or other inflammatory signs are dominant (i.e., reflux laryngitis), acid secretion
suppression or blockade are the mainstay, and endoscopy (with biopsy) offers the best
diagnostic discrimination. These measures typically prompt subspecialist assistance.
Infantile reflux typically presents with overt regurgitation and dyspepsia (colic).
These can be expected to improve markedly over the first year of life with the
transition to a diet based more on solids than liquids and attainment of a more upright
posture. Conversely, GE reflux in the older child tends to present as chronic or
recurrent pain, with only secondary signs or symptoms of reflux and no overt
regurgitation. It represents a chronic problem, the symptoms of which may run lifelong, and if mechanical measures and intermittent acid neutralization do not provide
adequate symptomatic relief, long-term medical therapy may be warranted. In either
case, in the absence of life-threatening complications, surgical options are not a
routine consideration, and generally are considered only in the face of failure of
extended and aggressive medical management of significant levels of disease.
Questions
1. True/False: Gastroesophageal Reflux is a rare phenomenon in childhood.
2. For the vomiting infant:
a. The parents can be reassured it is a process the child will outgrow as they
get older.
b. Thickening the feedings sometimes works.
c. Proper positioning may be helpful.
d. Deserves further evaluation.
3. A one month old second born female presents with worsening of her GE reflux.
The regurgitation remains effortless, but is increasing in volume and seems more
prominent an hour or so after meals. She has been more demanding of feedings and
has had fewer wet diapers over the last few days and is losing weight. Her parents
have felt "something moving" in her stomach in the hour after feedings over the last
week. What is happening?
4. True/False: A 4 year old with complaints of abdominal pain that disrupt school
attendance warrants a two week trial of a proton pump inhibitor.
5. True/False: A diagnosis of pain due to gastroesophageal reflux is likely to lead to
a lifetime of expensive medication.
References
1. Rudolph CD, Mazur LJ, Liptak GS, et al. Pediatric GE Reflux Clinical
Practice
Guidelines. J Pediatr Gastroenterol Nutrition 2001;32(suppl 2):S1-S31.
Answers to questions
1. False. Though most episodes are asymptomatic, reflux is a routine
physiologic phenomenon in everyone, at every age. It is gastroesophageal reflux
DISEASE that is uncommon in most of childhood.
2. d. Remember regurgitation is effortless, vomiting is forceful and is
atypical for uncomplicated GE reflux. It can indicate obstruction or metabolic
derangement, and represents a problem that requires an answer in as short a period of
time as possible (even if the answer is a diagnosis of routine gastroenteritis).
3. Consider pyloric stenosis, even if only a few of the classic symptoms and
signs are present. Waiting for the diagnosis to become more obvious further delays
surgical intervention and increases the risk of complications such as hypochloremic
alkalosis and dehydration. See differential diagnosis above.
4. This one is arguable, but my personal preference is to start treatment with
antacids since it offers a means of immediate relief of any truly peptic pain episode,
and younger children are better reinforced by immediacy of the response. Of course a
good history and physical should come first to verify the pain does fit a "peptic"
pattern, as constipation is more likely at this age.
5. False. The vast majority of uncomplicated pain seems to respond to
mechanical measures, avoidance of caffeine, nicotine, and the like, and intermittent
antacid use. It is only when the pain episodes remain disruptive more than once
weekly that it is generally warranted to proceed to chronic medical therapy, and then
only at the minimal doses necessary unless other complications (e.g., Barrett's
esophagus) occur.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
Malabsorption:
A 15 year old girl presents to the physician's office with a three year history of
intermittent diarrhea. Further history reveals a past history of anemia, anorexia, and
minor abdominal pain. Her weight has been the same for 3 years now. Her mother
has attributed this to her having a "rough time in school". Her mother also questions
whether the symptoms could be related to a recent move from their home state of
Minnesota. She has not yet reached menarche. A diet history suggests a normal diet
with adequate iron intake. Her family history is negative for malabsorption and
inflammatory bowel disease.
Exam: VS T 37.5, P98, R 18, BP 110/70. Ht 145 cm (57 inches), wt 35 kg (78
pounds), both less than the 5% percentile. She is alert, active and cooperative. She is
thin and small for age but not cachectic. HEENT: Her conjunctivae are pale. Her
teeth are pitted and discolored. Her neck is supple without adenopathy. Her heart and
lungs are normal. Her abdomen is slightly protuberant and hyperresonant. Bowel
sounds are slightly hyperactive. Tanner stage 4 for both breasts and pubic hair.
Examination of both hands reveal mild pallor and flat fingernails. She is able to
ambulate and no neurologic deficits are noted.
Because of her small size and amenorrhea, a bone age reveals a 3 year delay and
suggests osteopenia. A stool examination is negative for blood and reducing sugars.
The 72-hour fecal fat study shows a moderate increase in fat content. A CBC shows a
mild anemia. Her reticulocyte count is low and her iron studies indicate the presence
of iron deficiency. An ESR is normal, making the possibility of inflammatory bowel
disease less likely. Her small size, the steatorrhea and the iron deficiency all suggest
the possibility of some type of GI malabsorption condition. An upper GI study with
small bowel follow-through is normal. A chloride sweat test for cystic fibrosis is
normal. A lactose breath hydrogen test showed an elevation in hydrogen of 40 ppm,
suggesting carbohydrate malabsorption. An upper GI endoscopy shows no visible
abnormalities; specifically, no ulcers or hemorrhage. Biopsies from the duodenal and
proximal jejunal area reveal severe villus atrophy consisting of a flat mucosa with
deep crypts and no evidence of Giardia lamblia. A serologic human anti-tissue
transglutaminase ELISA test came back positive for autoantibodies suggesting the
diagnosis of celiac disease.
She is started on a strict gluten-free diet. She responds dramatically and upon followup is now reporting an increased appetite and improved mood. She has remained
symptom free for about a year now. She has also noticed a resurgence in her growth
and has reported menarche that started about a month ago.
Gluten-sensitive enteropathy, also known as sprue, celiac sprue and celiac disease, is
one of the many causes of malabsorption. Malabsorption is a clinical term for the
entire spectrum of conditions occurring during digestion and absorption of ingested
nutrients by the gastrointestinal tract. Perturbations in the digestion and absorption of
food nutrients can occur either in the luminal phase, the mucosal phase, or the
transport phase of the ingested food. Classifying the many entities of malabsorption
in this manner makes it easier to understand their exact mechanisms. Causes of
malabsorption can be explained by the way the disease process interferes with the
normal digestive and absorptive mechanisms.
Malabsorption encompasses conditions that go from a single nutrient malabsorption
(e.g., lactose intolerance) to pan malabsorption (e.g., cystic fibrosis and celiac sprue).
Celiac sprue is a disease that predominantly affects people of European descent (rare
in people of African and Asian ethnicity), and currently has a frequency of 1 in 5000
in the US (although this frequency is debated and some feel that this frequency may
be much higher). Celiac disease is manifested variably by malabsorption to different
types of nutrients. While presenting symptoms such as diarrhea and weight loss are
common, the specific cause of malabsorption should be established using
physiological evaluations. In celiac sprue, suggestive screening investigations include
steatorrhea that denotes fat malabsorption, decreased d-xylose absorption found in
carbohydrate malabsorption, and serological markers such as antigliadin antibodies or
the new ELISA test for anti-tissue transglutaminase. The treatment of the underlying
disease is often dependent on the establishment of definitive cause for the
malabsorption. In the case described, gluten-sensitivity is the underlying cause.
Were it not for the logical steps followed by the clinician, the quite dramatic
resolution of symptoms brought about by denying the patient food devoid of gluten
would not have happened if the exact etiology was missed.
As emphasized before, the causes of malabsorption can be best appreciated if they are
classified into the specific phase of digestion and absorption that is disturbed.
1. The luminal phase is where dietary fats, proteins, and carbohydrates are
hydrolyzed and stabilized by digestive enzymes and bile. Diseases often associated
with this phase include:
- Enterokinase and trypsinogen deficiencies that can lead to protein
malabsorption.
- Impaired micelle formation that can cause problems in fat stabilization and
the resulting fat malabsorption due to deconjugation of bile salts in bacterial
overgrowth.
- Stasis of intestinal content due to a variety of factors (motor and anatomic
abnormalities, small bowel contamination from enterocolonic fistulas) can cause
bacterial overgrowth.
- Bacterial overgrowth can also cause decreased luminal availability of
substrates (carbohydrates, protein, vitamins).
2. The mucosal phase relies on the integrity of the brush border membrane of
intestinal epithelial cells to transport digested products from the lumen into the cells.
- Impaired brush border enzyme activity may lead to lactose intolerance and
sucrase-isomaltase deficiency.
- Impaired nutrient absorption can be inherited or acquired deficits. Inherited
defects include glucose-galactose malabsorption, a-betalipoproteinemia, and Hartnup
disease.
- Acquired defects which are more common may be caused by decreased
absorptive surface area (intestinal resection), damaged mucosa (celiac sprue, tropical
sprue, giardiasis, Crohn's), or an infiltrating disease of the intestinal wall (lymphoma,
amyloidosis).
- Tropical sprue and Giardiasis are two mucosal phase abnormalities due to
damaged intestinal mucosa. Tropical sprue, a syndrome characterized by diarrhea,
weight loss, and malabsorption, occurs in residents or visitors to the tropics and the
subtropics, usually in connection with certain geographical areas such as Southeast
Asia and the Caribbean. The pathophysiology is poorly understood but is theorized to
be caused by an acute intestinal infection that leads to jejunal mucosal injury. The
disease is primarily a disease of adults but it is also described in children.
- Giardiasis like celiac sprue has symptoms characteristic of
panmalabsorption. The organism, Giardia lamblia is a protozoan that appears to alter
intestinal epithelial structure and function leading to malabsorption. Giardiasis
usually begins with ingestion of the cyst that eventually leads to trophozoites in the
stomach and duodenum. High-risk groups include travelers, homosexual men,
individuals with immunoglobulin deficiency states, and children, especially those who
attend day care centers.
3. Transport (removal) phase malabsorption abnormalities may be caused by
lymphatic obstruction or vascular insufficiency.
The signs and symptoms of malabsorption depend on the specific cause or etiology,
and the specific nutrient deficiency that ensues. Age also plays a big factor.
Malabsorption causes a far more acute and wide-ranging symptomatology in younger
children than in the older child. Diarrhea is the most common symptomatic complaint
which might lead to dehydration, especially in younger patients. In older children,
weight loss and fatigue might be more pronounced. Some patients may try to
compensate by increasing caloric consumption, making the diagnosis more difficult.
Children in the pubertal ages may display disturbance in anthropometric growth
(weight, height, weight by height) and pubertal development. Other symptomatology
that follows from specific components of disturbed digestion and absorption include:
Steatorrhea: Most often due to fat malabsorption.
Flatulence and abdominal distention: Release of gas by bacterial fermentation
of undigested or unabsorbed food particles.
Edema: Commonly from chronic protein malabsorption or loss of protein into
the lumen.
Anemia: Depending on the cause, can be micro or macrocystic, such as Fe
deficiency anemia secondary to celiac disease.
Bleeding disorders and ecchymosis:
Usually a result of vitamin K
malabsorption.
Bone defects: Can be due to vitamin D deficiency or calcium malabsorption.
Neurological manifestations: Electrolyte disturbance or specific vitamin
malabsorption such as B12 neuropathy.
A malabsorption condition usually contains a specific physical manifestation
(e.g., growth, weight, and pubertal disturbance). Growth and developmental charts
are extremely helpful in diagnosis. A physical examination should look into the
possibility of finding specific signs of individual nutrient malabsorption to aid in the
organization of symptoms.
General: Orthostatic hypotension, weight loss, muscle wasting, loss of
subcutaneous fat, pallor, ecchymosis.
HEENT: Alopecia or thinning hair, pale conjunctiva, aphthous ulcers,
cheilosis, glossitis, dental hypoplasia, abdominal distention, tympanic abdomen,
hyperactive bowel sounds, ascites.
Hands: Koilonychias (flattened or spoon shaped nails), pale skin.
Neurologic: Motor weakness, peripheral neuropathy, ataxia, Chvostek or
Trousseau's sign due to hypocalcemia or hypomagnesemia, numbness, paresthesias.
Musculoskeletal: Mono or polyarthropathy, back pain, muscle weakness.
After the initial examination, a physician may have a reasonable idea of a diagnosis.
Often, laboratory investigations are needed to confirm the cause.
Stool analysis: Quantitative stool fat analysis for 72 hours, consistency, pH (due to
acidic stools in the presence of fermented sugars and reducing sugars in carbohydrate
malabsorption), stool bile acids (increased in bacterial overgrowth syndromes),
presence of large serum proteins (such as alpha-1-antitrypsin, may indicate a proteinlosing enteropathy, ova and parasites (for Giardia), testing for chronic intestinal
infections (such as C. difficile or Cryptosporidium), consistency, fecal leukocytes
(such as in inflammatory bowel disease), and occult blood.
Screening Lab Tests: CBC (for anemia), blood smear (for abnormal blood
morphologies that may indicate vitamin B-12 deficiency, amyloid, or abetalipoproteinemia), urinalysis (urine may reveal unusually high concentration of
malabsorbed substance since, in many cases, the kidney and the gut use the same
transporter such as in glucose transporter deficiencies where urinary glucose will be
elevated while the serum level is normal), total protein (decreased in protein
malabsorption), albumin (decrease may indicate severe malnutrition or pancreatic
insufficiency), liver function tests (in liver and biliary diseases), calcium and
phosphorus levels (to determine vitamin D deficiency which might be present in gross
steatorrhea), serum immunoglobulins (for autoimmune enteropathies), sweat test (for
cystic fibrosis), and radiographic bone age (especially in pediatric patients).
Other lab studies to be considered include: Schilling test (for B12 deficiency),
urinary 4-hydroxy phenylacetic acid (shown to be elevated in the urine of children
with bacterial overgrowth syndrome; enteric bacteria that posses L-amino acid
decarboxylase produce 4-hydroxyphenylacetic acid from dietary tyrosine), fat-soluble
vitamin assays for fat malabsorption, LDL cholesterol (may be lower than normal
with bile and malabsorption), ESR and C-reactive protein (may be elevated in
inflammatory bowel disease), serum bile acids (congenital deficiency in the sodiumbile cotransporter results in primary bile acid malabsorption, which may appear as
diminished reabsorption of bile acids in the kidney), GI motility studies (can be as
simple as an upper GI contrast study or as complex as the performance of
antroduodenal motility studies, depending on the need, which can recognize damage
or abnormalities present in the gut due to inflammatory and infective processes),
breath hydrogen analysis and, d-xylose absorption study (to specify carbohydrate
malabsorptions), carbohydrate oral tolerance test (for specific carbohydrate digestion
and absorption dysfunctions). IgG and IgA antigliadin and IgA antiendomysial
antibodies are present in gluten-sensitive enteropathy. The presence human antitissue
transglutaminase ELISA has a reported sensitivity of 96-100% and specificity of 99100% and now appears to be the new criterion standard for screening of sprue.
Barium radiographic studies (upper GI series) can be useful in assessing anatomy and
function. Endoscopy performed by a gastroenterologist permits direct visualization of
the mucosal surface. During the endoscopy procedure, mucosal biopsies can provide
histological information, identification of infective organisms and functional assays of
the biopsied tissue for specific enzymes.
Medical management of underlines two basic principles: 1) Treatment of the etiology
of malabsorption. 2) Correction of nutritional deficiencies.
Treatment of malabsorption is as varied as the etiologies that cause them. The
intestine appear to repair itself slowly, thus treatment may require a longer course.
Since many of the conditions that are caused by malabsorption, respond well to
specific remedies, the need to make an accurate diagnosis becomes even more
important. Most treatments highlight the dramatic effect of correcting the underlying
defects of digestion and absorption. An example would be the gluten-free diet
instituted in patients with gluten sensitivity. The effects of strict adherence to this diet
include the full reversal of the disease process. Treatment of diarrhea with oral
gentamicin or an appropriate broad-spectrum antibiotics that includes anaerobic
coverage (e.g., metronidazole) reverses bacterial overgrowth and chronic infections,
resulting in a rapid improvement in the patient's function. Specific malabsorption
syndromes that include enzyme deficiency or imbalance can be successfully managed
with oral supplements. The use of lactase supplements or non-lactose containing milk
substitutes is beneficial in lactose intolerance. Pancreatic lipase can be replaced with
oral supplements to improve fat digestion.
Efficient absorption of essential nutrients will not occur if the diarrhea is still present.
Usually, the complete withdrawal of the offending substance can correct diarrhea.
Care should be taken to avoid the iatrogenic elimination of necessary nutrients. For
example, in celiac disease, gluten withdrawal is often enough to correct the
symptoms. Carbohydrates and other foodstuff not continuing gluten can continue to
be consumed.
Correction of nutritional deficiencies is the other important treatment goal. Dietary
modifications and supplementation are especially useful if one considers the slow
self-repair process of the severely damaged intestines. Dietary changes should be
individually tailored to the individual and the underlying cause of malabsorption, but
in general, a high protein, low fat diet is recommended. Decreased fat intake also
reduces steatorrhea. The dietary modifications closely parallel the essence of
withdrawing certain offending food products in the diet and promoting adequate
calorie intake. Vitamin, mineral, and trace element deficiencies should be sought and
corrected. Supplementation of nutrients whose absorption and digestion mechanisms
were disturbed is essential. Anemia should be treated with appropriate supplements
and specific deficiencies corrected by oral (or parenteral) supplementation. Fatsoluble vitamins may be required for a patient with severe steatorrhea.
Questions
1. What are the three phases of digestion and absorption discussed in this
chapter?
2. Dietary fats, proteins, and carbohydrates are hydrolyzed and stabilized by
digestive enzymes and bile in which phase?
3. Identify the phase in which digested food is moved from the lumen into the
cells.
4. True/False: The symptoms of malabsorption are worse in older children
compared to younger children.
5. True/False: Diarrhea is the most common presenting symptom of
malabsorption in younger children.
6. True/False: Withdrawal of gluten-containing food from a patient with
celiac disease is often enough to reverse the symptoms of malabsorption.
References
1. Riley SA, Marsh MN. Chapter 88 - Maldigestion and Malabsorption. In:
Feldman M, et al. (eds). Sleisenger and Fordtran's Gastrointestinal and Liver Disease,
sixth edition. 1998, New York: W.B. Saunders Company, pp. 1501-1520.
2. Yang VW. Malabsorption. eMedicine Journal 2001 September;2(9).
3.
Frye RE.
Malabsorption Syndromes.
eMedicine Journal 2001
September;S(11).
4. Talusan-Soriano K, Lake A. Malabsorption in Children. Pediatr Rev
1996;17(4):135-142.
5. Busschots GV. Sprue. eMedicine Journal 2002 January;3(1).
6.
Godkin A, Jewell D.
The pathogenesis of Celiac Disease.
Gastroenterology 1998;115(1):206-210.
7. Bai J. Malabsorption Syndromes. Digestion 1998;59(5):530-546.
8. Lindley KJ, Macdonald S. Malabsorption in Children. Practitioner
2001;245(1620):169-170.
Answers to questions
1. Luminal phase, mucosal phase, and transport (removal) phase.
2. Luminal phase.
3. Mucosal phase.
4. False. Younger patients often display a more acute and wider-ranging
symptomatology than older children.
5. True.
6. True.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
Neonatal sepsis:
This is a 3200 g term newborn female delivered via normal spontaneous vaginal
delivery to a 25 year old G1P0 syphilis non-reactive, group B strep (GBS) negative,
rubella immune, hepatitis B surface antigen negative mother with early preeclampsia
and thrombocytopenia (platelet count 80,000). Rupture of membranes occurred 11
hours prior to delivery with clear fluid. Intrapartum medications included 3 doses of
butorphanol (narcotic opioid analgesic). The last dose was administered within 1 hr
of delivery. There was no maternal fever. Apgars were 8 and 9.
In the newborn nursery, vital signs are: HR 140, T 37, BP 47/39, RR 54. Oxygen
saturation is 98-100% in room air. The infant appears slightly pale and mottled. She
is centrally pink with persistent grunting, shallow respirations, and lethargy. Her
fontanelle is soft and flat. Heart exam is normal. Lungs show good aeration.
Abdomen is soft and without masses. Pulses are 1+ throughout with 3-4 sec capillary
refill. Neuro exam shows decreased tone and a weak, intermittent cry.
Labs: CBC with WBC 3,200, 6% segs, 14% bands, 76% lymphocytes, Hgb 15, Hct
43, platelets 168,000. Blood glucose 52. The chest x-ray is rotated with fluid in the
right fissure, diffuse streakiness on the left, and a normal cardiac silhouette. CBG
(capillary blood gas) pH 7.31, pCO2 43, pO2 44, BE-4. CSF: 2430 RBCs, 20 WBCs,
1% PMN, 17% lymphs, 82% monos, glucose 39, protein 133, gram stain shows no
organisms.
You are asked to consult on this case. What other tests would you obtain? What
would your assessment be for this infant? What would your recommendations be (if
any) for further evaluation or treatment? If you were to treat this infant, how long
would you treat her?
The evaluation and management of the neonate at risk for sepsis is potentially a
source of frustration for students and practitioners. The convention in the past has
often been to evaluate and empirically treat all neonates felt to be at significant risk,
especially as relates to maternal factors and the receipt of maternal antibiotics in
labor. Due to evolutions in health care and the advent of intrapartum prophylaxis for
group B streptococcal sepsis (mothers are routinely screened for group B strep and if
found to be positive, they are given ampicillin prior to delivery), more attention has
come to focus (very appropriately) on the clinical evaluation of the infant as a major
part of the decision to evaluate and treat with antibiotics. This factor; however,
remains fraught with a degree of uncertainty related to the nonspecific manifestations
of infection in the newborn, the sometimes rapid progression of sepsis in the newborn,
and the lack of laboratory tools which have high positive predictive accuracy.
The approach in this section of neonatal sepsis will be to: 1) incorporate the
evolutionary changes in management which are based on more recent evidence; 2) to
emphasize the lack of a gold standard underlying the variations in practice (i.e.,
clinical sepsis with a negative blood culture is still more often diagnosed than blood
culture proven sepsis); and 3) to suggest (based on interpretation of older and recent
evidence) newer concepts which place more reliance on tests with high negative
predictive accuracy and the efficacy of intrapartum antibiotics (1-7).
The information upon which former standard practice is based is also provided
throughout the chapter. These are necessary and basic to understanding the problem
of neonatal sepsis and perinatal infections. However, the evaluation and management
will de-emphasize empiric treatment for risk alone, and variation in practice will be
seen as a necessary consequence of our lack of knowledge and the inherent variation
in individual practitioner's tolerance of degree of risk and uncertainty.
Table 1. Common bacterial and viral infectious agents causing sepsis (or something
similar to sepsis):
E. Coli
Group B streptococcus (GBS)
Listeria monocytogenes
Herpes simplex
Cytomegalovirus
Any virus infecting the mother in the week prior to delivery any bacteria
cultured from the mother on admission for labor
Table 2. High risk factors for neonatal sepsis:
Late maternal prenatal care
Maternal UTI, STD, or abnormal serologies
Prolonged rupture of membranes (>24 hrs)
Maternal fever prior to delivery
Maternal chorioamnionitis
Prematurity
GBS positive screen without intrapartum prophylaxis
Table 3. Signs and symptoms of neonatal infection (most are NONSPECIFIC):
Apnea and dusky episodes for no clear reason.
Lethargy, poor color, hypoactivity, poor capillary refill.
Feeding intolerance (more than usual spit-up), abdominal distention.
Clinical appearance; doesn't look "good".
Tachypnea, temperature instability, look of distress.
Table 4. The most important risk factors for neonatal sepsis:
Prematurity
Untreated maternal chorioamnionitis.
Untreated maternal prolonged rupture of membranes.
Maternal fever, untreated.
Untreated positive maternal GBS screen.
Table 5. Equivocal risk factors (i.e., they overlap or may result in similar
manifestations):
Fetal distress.
Depression at birth (needs resuscitation, low 5 minute Apgar).
Meconium staining.
Hypoglycemia.
Any unusual finding which may be due to infection.
Although we have gained more knowledge about risk factors and have more
antibiotics at our disposal, there is still NO GOLD STANDARD for the diagnosis of
neonatal infection. There are still many unknowns in neonatal sepsis which continue
to elude us, and compel the diagnosis of neonatal infection to be made clinically more
often than not.
Table 6. The Unknowns in Neonatal Sepsis:
1. How effective is GBS prophylaxis as prescribed? >95%
2. How sensitive are blood cultures (i.e., how often are they positive) ?
3. Can an elevated l/T ratio (immature to total granulocyte ratio) indicate
acute OR resolving inflammatory response?
4. Will ampicillin resistant organisms be seen with more use of intrapartum
ampicillin prophylaxis?
5. What is the minimum duration of antibiotic treatment to effectively treat
sepsis?
6. Is neonatal infection with a positive blood culture the same as neonatal
sepsis?
7. When does neonatal sepsis become SIRS (systemic inflammatory response
syndrome), i.e., overwhelming sepsis?
8. What is the immunologic competence level of a given infant at risk (i.e.,
will the infant be able to respond positively with appropriate antibiotics)?
9. Does intrapartum treatment of the mother for chorioamnionitis also treat
the fetus effectively?
Because we have many unknowns and the worst case scenario for neonatal
infection is sepsis and perhaps overwhelming sepsis or death from SIRS, pediatricians
have tended to err on being conservative in the evaluation for sepsis. This intention
paradoxically results in a more "aggressive" approach to the patient in terms of tests
and/or treatment. This paradox is underscored by the lack of a gold standard for
diagnosing sepsis in the newborn, and complicated by the recent increase of
intrapartum antibiotics prescribed to women in labor.
Table 7. The full sepsis work-up.
1. CBC differential, platelet count.
2. Blood, urine, and CSF cultures.
3. CXR. Add a tracheal aspirate for gram stain and culture if the patient is
intubated.
4. Equivocal: gastric aspirate for gram stain and culture.
5. Start broad spectrum antibiotics while awaiting culture results.
For a partial sepsis work-up, one could pick any one or more of the above
items. For a totally asymptomatic infant with high risk factors, none of the steps
might be elected (practice variation). This is based on the premise that the clinical
appearance and serial monitoring of the infant is just as accurate as any laboratory test
for indicating the presence of infection, given any set of risk factors in an infant with
a relatively normal exam.
This wide variation of practice suggests that the unknowns in neonatal sepsis (see
above) are quite important to practical management. This may lead one to be more or
less restrictive in practice, and requires one to have thorough knowledge of the
predictive accuracy of the objective tools available in the assessment of neonatal
sepsis. From an outcomes point of view, one would expect that if certain practices
were inappropriate, there would be a higher rate of readmission within two weeks of
discharge from the normal nursery for those regimens which were "least restrictive."
Such evidence has not emerged from this institution, based on a review of early
discharge from the nursery in the mid-1990's, when the most common cause for
readmission was jaundice (infection and sepsis were not found).
The highest degree of controversy surrounds the group of infants who are
asymptomatic with some risk factors for sepsis, especially those whose mothers
received intrapartum antibiotics. In these infants, there is the fear of partially treated
sepsis, prompting evaluation and treatment of these infants based on their risk factors
and discounting the maternal antibiotics. However, the asymptomatic state could also
be interpreted as adequate prophylactic treatment for neonatal bacteremia. In 1990,
Wiswell et al., reported on a survey of academic infectious disease departments with
respect to management of this scenario. They concluded that there is no consensus
regarding management of pretreated, healthy appearing, term gestation neonates (8).
In contrast, Teji et al (1994) surveyed neonatologists in Midwestern states of the U.S.
with regard to the management of PROM (prolonged rupture of membranes) without
chorioamnionitis, chorioamnionitis without treatment prior to delivery, and
chorioamnionitis with treatment prior to delivery. One hundred thirty seven responses
were received and prematurity and severity of maternal illness significantly
influenced the decision to treat empirically, irrespective of screening test results (9).
More recently, Eichenwald (1997) has suggested a very reasonable scheme for
evaluation of the asymptomatic term infant, based on a protocol developed by the
Joint Program in Neonatology in Boston (Table 8) (10). However, the question
persists and evolves regarding the benefits and risks of routine therapy of high risk
neonates vs. clinical observation and selective therapy of only those infants who
manifest symptoms. This evolution is highlighted by the recent reports of ampicillinresistant organisms in neonatal sepsis (11-13) and the dramatically increased
incidence of Candida species sepsis in very premature infants in NICU settings over
the last decade, of which one very important contributor is the prior use of antibiotics.
Table 8. Management of asymptomatic term infants with risk factors for infection for
term, well appearing infants with maternal antibiotics given in labor.
Tests: CBC, differential, platelet count, blood culture (volume of blood is
important; 1cc recommended).
Antibiotics until 48 hour blood culture results are available if:
l. I/T>0.2 (immature neutrophils to total neutrophil ratio) or
2. WBC <5000 or
3.
Mother received antibiotics for suspected or diagnosed
chorioamnionitis.
4. GBS positive mother, adequately treated, but with a history of a
previously affected infant with invasive GBS infection
In this scenario, the availability of rapidly available tests with high negative predictive
accuracy would seem particularly useful. Several fitting this category are:
1. The total WBC and l/T ratio (14)
2. Serial CBC's using total WBC and l/T ratio (15)
3. A hematologic scoring system (16)
4. A combination of CRP and IL-6 (17)
Singhal and La Gamma (1996) in a study of 6620 pregnancies, found that 82% of atrisk patients are asymptomatic and have negative body fluid cultures. They
concluded that their data support restricting a full course of antibiotic treatment to
only those patients with clinical or laboratory signs of sepsis (18%) (5). Escobar et al
(2000) reported on a large population of newborns in the Kaiser system of whom 15%
were evaluated for sepsis (4). Only 2.2% met criteria for proven, probable, or
possible bacterial infection, and of those meeting criteria, 0.8% had positive cultures
while 1.4% had clinical evidence of bacterial infection. 1568 out of 2785 infants
evaluated were not treated, and the initial asymptomatic status was associated with a
decreased risk of infection (adjusted odds ratio; AOR=0.26). Factors associated with
increased AOR for infection were chorioamnionitis, low absolute neutrophil count,
and meconium stained amniotic fluid. They concluded that evidence based
observation and treatment protocols could be defined, based on a limited set of
predictors: maternal fever, chorioamnionitis, initial neonatal examination, and
absolute neutrophil count (4). Ultimately, each practitioner must determine the
degree of risk or uncertainty that he or she can accept on the basis of clinical and
institutional experience.
Table 9. A revised and composite screen and evaluation for sepsis in the neonate.
Risk factors: Prematurity, chorioamnionitis, prolonged rupture of membranes,
maternal fever, fetal tachycardia and depression at birth.
Screening tests: CBC and differential and platelet count; total WBC and I/T
ratio (initial and serial); blood culture; urine culture or GBS antigen; others as may be
available to increase negative predictive accuracy such as CRP, IL-6; clinical exam of
the infant.
Completing the evaluation: Lumbar puncture for CSF studies and culture;
CXR and/or endotracheal aspirate for gram stain and culture.
Treatment: Institute broad spectrum antibiotic coverage for neonatal sepsis. If
meningitis is suspected, add cefotaxime to the regimen.
For premature infants in whom the physical exam may be more equivocal and
whose prematurity constitutes an additional risk factor, the threshold for a full sepsis
evaluation and antibiotic treatment is much lower.
Table 10. Rates of infection in neonates and mortality rates.
Proven sepsis: 2.5 per 1000 live births, mortality 8.7% (18).
Clinical sepsis: 3.6 per 1000 live births, mortality 4.3% (18).
VLBW (very low birth weight) proven sepsis: 26.5 per 1000 live births (18).
VLBW clinical sepsis: 32.4 per 1000 live births (18).
EOGBS (early onset GBS) 0.5 per 1000 live births, mortality 10% (19).
Other early onset infection 0.5 per 1000 live births (19).
Questions
1. For the case presented at the beginning of this chapter, you are asked to
consult on this case. What other tests would you obtain'?
2. What would your clinical assessment of this infant be?
3. What would your recommendations for further evaluation and/or treatment
be?
4. If you were to treat this infant, how long would you treat?
5. What tests have the highest positive predictive accuracy in neonatal sepsis?
6. What tests have the highest negative predictive accuracy in neonatal
sepsis?
7. Is the volume of blood obtained for the blood culture important to the
culture being positive or negative?
8. Is there good evidence that treatment of maternal chorioamnionitis prior to
delivery significantly reduces the risk of neonatal infection?
9. Does prophylaxis for group B strep infection alter the time course of early
onset group B streptococcal sepsis if prophylaxis is ineffective?
10. What is the incidence of neonatal sepsis and what is the mortality from
neonatal sepsis?
References
1. Gibbs KS, Dinsmoor MJ, et al. A randomized trial of intrapartum
antibiotic prophylaxis vs. immediate postpartum treatment of women with intraamniotic infection. Obstet Gynecol 1988;72:823-828.
2. Sperling KS, Ramamurthy KS, Gibbs KS. A comparison of intrapartum
vs. immediate postpartum treatment of intra-amniotic infection. Obstet Gynecol
1987;70:861-865.
3. Mecredy RL, Wiswell TF, Hume KF. Outcome of term gestation neonates
whose mothers received intrapartum antibiotics for suspected chorioamnionitis. Am J
Perinatol 1993; 10:365-8.
4. Escobar GJ, Li DK, et al. Neonatal sepsis workups in infants >/=2000
grams at birth: A population based study. Pediatrics 2000;106(2 Pt 1):256-263.
5. Singhal KK, La Gamma EF. Management of 168 neonates weighing more
than 2000 g receiving intrapartum chemoprophylaxis for chorioamnionitis.
Evaluation of an early discharge strategy. Arch Pediatr Adolesc Med 1996;150:158163.
6. Cararach V, Botet F, et al. Administration of antibiotics to patients with
rupture of membranes at term: a prospective, randomized, multicentric study.
Collaborative Group on PROM. Acta Obstet Gynecol Scand 1998;77:298-302.
7. Schrag SJ, Zywicki S, et al. Group B streptococcal disease in the era of
intrapartum antibiotic prophylaxis. N Engl J Med 2000;342:15-20.
8. Wiswell TE, Stoll BJ, Tuggle JM. Management of asymptomatic, term
gestation neonates born to mothers treated with intrapartum antibiotics. Pediatr Infect
Dis J 1990;9:826-831.
9. Teji JS, Srinivasan G, et al. Management of asymptomatic neonates with
prolonged rupture of membranes. Indian J Pediatr 1994;61:63-69.
10. Eichenwald EC. Perinatally transmitted neonatal bacterial infections. Inf
Dis Clin North Am 1997;11:223-239.
11. Levine EM, Ghai V, et al. Intrapartum antibiotic prophylaxis increases
the incidence of gram-negative neonatal sepsis. Infect Dis Obstet Gynecol
1999;7:210-213.
12. Mercer BM, Carr TL, et al. Antibiotic use in pregnancy and drug-resistant
infant sepsis. Am J Obstet Gynecol 1999;181:816-821.
13. Schuchat A, Zywicki SS, et al. Risk factors and opportunities for
prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics
2000;105(1 Pt l):21-26.
14. Sinclair J. Early diagnosis of neonatal sepsis. Yearbook of Pediatrics
1982:11-13.
15. Greenberg DN, Yoder BA. Changes the differential white blood cell
count in screening for group B streptococcal sepsis. Pediatr Infect Dis J 1990;9:886889.
16. Rodwell RL, Leslie AL, Tudehope DI. Early diagnosis of neonatal sepsis
using a hematologic scoring system. J Pediatr 1988;112:761-767.
17. Doellner H, Arntzen KJ, et al. Interleukin-6 concentrations in neonates
evaluated for sepsis. J Pediatr 1998;132:295-299.
18. Lopez Sastre JB, Coto Cotallo GD, et al. Neonatal sepsis of vertical
transmission: an epidemiological study from the "Grupo de Hospitales Castrillo." J
Perinat Med 2000;28:309-315.
19. Isaacs D, Royle JA. Intrapartum antibiotics and early onset neonatal
sepsis caused by group B streptococcus and other organisms in Australia.
Australasian Study Group for Neonatal Infections. Pediatr Infect Dis J 1999;18:524528.
20. Bromberger P, Lawrence JM, et al. The influence of intrapartum
antibiotics on the clinical spectrum of early onset group B streptococcal infection in
term infants. Pediatrics 2000;106:244-250.
Answers to questions
1. Blood and urine cultures, if not already done.
2. Clinical sepsis with poor perfusion and neutropenia; possible septic shock
with narrow pulse pressure.
3. a) Repeat CBC to monitor the neutropenia and thrombocytopenia. b)
Volume bolus to improve perfusion. c) Follow-up exam of abnormal tone and cry
after instituting supportive therapy. d) Start broad spectrum antibiotics parenterally.
e) Transfer from the normal nursery to a higher level nursery or intensive are unit for
continuous monitoring of vital signs.
4. Seven to ten days empirically, given the clinical presentation and
depending on culture results. Serial CRPs may also be used to assist with duration of
treatment.
5. Any 2 from the battery reviewed by Sinclair (14) gave 62% for sepsis
proved or probable.
6. Again any 2 from the above reference (14) gives 98% negative predictive
accuracy for sepsis proved or probable. However, the CBC and differential alone will
give you two out of this battery.
7. Yes. At least one ml should be obtained for blood cultures.
8. Yes
9. No. This has implications for the current AAP protocol for monitoring
infants whose mothers did not receive prophylaxis.
10. 2.5 per 1000 live births, with mortality rate of 8.7% (18). Clinical sepsis
is cited as 3.6 per 1000 live births with mortality of 4.3%. Figures are much higher for
VLBW infants.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
Neonatal jaundice:
Case 1
This is a jaundiced, 4 day old, 3.1 kg, appropriate for gestational age (AGA)
Asian female infant born at term to a 25 year old A+ primiparous woman with
gestational diabetes. The pregnancy was otherwise uneventful. Labor was
augmented with Pitocin. The baby was discharged home on day of life 2 at which
time her weight was down 4% from birth weight and she had mild facial jaundice. In
the hospital, she was breast fed every 3 hours and had 2 wet diapers and one
meconium stool over a 24 hour period. On day 3, her parents gave her water on two
occasions as she appeared hungry despite regular and frequent breast feeding
attempts. In addition, they noted an increase in the degree of jaundice, but failed to
address it after being reassured by family members that jaundice is common. They
also had an appointment to see their pediatrician the following day. In the office, on
day 4, mother reports that she is breastfeeding the baby every three hours and that
there have been 2 wet diapers per day. The urine is described as dark yellow in color
and the stools appear dark green.
Exam: VS T 37.8, P 162, RR 55, BP 63/45. Weight 2.7 kg (25%ile), length 50 cm
(75%ile), head circumference 34 cm (75%ile). The infant is jaundiced and irritable.
The anterior fontanel is slightly sunken, the oral mucosa is tacky, and there is jaundice
to the lower extremities. No cephalohematoma or bruising is present. The sclera of
both eyes are icteric. Muscle tone and activity are normal. The remainder of the
physical exam is normal.
The total bilirubin is 20 mg% with a direct fraction of 0.7 mg%. She is admitted to
the hospital for phototherapy, supplementary formula feedings, and lactation
consultation. By the following day, the bilirubin has decreased to 12 mg% and she is
discharged home on breast milk feedings. The baby is scheduled for follow-up with
both the pediatrician and the lactation consultant.
Case 2
A 4 day old, 36 week gestation male presents to his primary care physician with
worsening jaundice. The maternal blood type is 0+. He was discharged home on day
2 of life after successfully breastfeeding for a 24 hour period. At the time of
discharge, his physical exam was remarkable for mild jaundice and a
cephalohematoma. At today's visit there is an 8% weight loss from birth and a history
of "fair" urine output and yellow stools. He is markedly jaundiced and has a resolving
cephalohematoma. Other physical exam findings are remarkable for a normal cry, flat
anterior fontanelle, moist oral mucosa and a normal neurologic examination. The
total bilirubin is 27 mg% with a direct fraction of 1 mg%. He is admitted to the
hospital where phototherapy is initiated. His blood type is A+ with a positive direct
Coombs. The hematocrit is 42% with a reticulocyte count of 12% and the pathologist
identifies spherocytes on the blood smear. The G6PD is pending. After one hour of
phototherapy, a repeat bilirubin is 25 mg%. The G6PD is normal. The decision is
made to perform a double volume exchange transfusion. The infant remains on
phototherapy for an additional 2 days and is discharged home after being off
phototherapy for 1 day. The serum bilirubin on the day of discharge is 12 mg% and
he passes an auditory brainstem response test.
More than 50% of term neonates become jaundiced (1). In this community (Hawaii),
hyperbilirubinemia is one of the major reasons for re-hospitalization within the first
two weeks of life. The primary reason for the level of concern over jaundice and
hyperbilirubinemia in the newborn is the association of hyperbilirubinemia with
kernicterus, which is a rare, but devastating neurologic complication of
hyperbilirubinemia. Kernicterus can occur without signs and symptoms (2), but acute
kernicterus in term babies is usually characterized by changes in muscle tone,
drowsiness, poor feeding, a high pitched cry, apnea, possible seizures, fever, and
death (3). Neurologic sequelae include dystonia and athetosis, upward gaze
abnormalities, sensorineural hearing loss, intellectual deficits and tooth enamel
dysplasia (3). In term patients, the MRI has increased signal intensity in the globus
pallidus on T2 images (2). Although kernicterus is rare, it is potentially preventable
and it is being seen with increasing frequency. This has prompted a recent Joint
Commission on Accreditation of Healthcare Organizations Sentinel Event Alert (4).
The assessment and management of hyperbilirubinemia can be confusing. For
instance, a bilirubin of 11 mg% has a different significance under different
circumstances. It would be considered physiologic (not pathologic) in a 4 day old
term breast fed baby, while the same level would be pathologic on day 1. A serum
bilirubin of 11 mg% would also be concerning in a sick, two day old, 27 week
gestation premature infant. Clinical decision-making is based on serum bilirubin
values which are not directly reflective of risk for neurotoxicity (3). In an attempt to
assist physicians with this common problem, the AAP released a practice parameter in
1994 on the Management of Hyperbilirubinemia in the Healthy Term Newborn (5).
According to the commentary from the AAP Subcommittee on Neonatal
Hyperbilirubinemia in 2001, this practice parameter is currently being revised (6).
Hyperbilirubinemia is more common in neonates due to the shortened life span of
their red blood cells, declining hematocrit, immature liver uptake and conjugation of
bilirubin, and increased intestinal reabsorption of bilirubin. Hemoglobin breakdown
releases iron, carbon monoxide, and biliverdin. The latter is reduced to bilirubin,
which enters the liver. Uridine diphosphate glucuronyltransferase (UDPGT)
conjugates bilirubin into an excretable form. Intestinal bacteria can deconjugate
bilirubin allowing for reabsorption of bilirubin into the circulation. This increased
enterohepatic circulation occurs particularly in preterm neonates with diminished
stool passage. In Asians, a variant in the UDPGT has been associated with
hyperbilirubinemia.
Infants with red blood cell membrane G6PD (glucose-6-phosphate dehydrogenase)
deficiency have a tendency for hemolysis and hyperbilirubinemia. In G6PD
deficiency, hyperbilirubinemia can occur despite minimal evidence for hemolysis.
Also, decreased conjugation of bilirubin has been described in G6PD deficiency.
Most unconjugated bilirubin is bound to albumin, but free unconjugated bilirubin (a
form unbound to albumin) can enter the brain (i.e., it can cross the blood brain
barrier). Sulfonamides are contraindicated in the neonatal period because they
displace bilirubin from albumin. Conditions that disrupt the integrity of the blood
brain barrier, such as infection (e.g., sepsis, meningitis, congenital viral infections),
acidosis, prematurity, and hyperosmolarity, place the infant at increased risk for
kernicterus. Hemolytic causes of hyperbilirubinemia (e.g., Rh incompatibility and
G6PD deficiency) have higher risks of kernicterus compared to other causes of
jaundice at comparable bilirubin levels.
Bilirubin may be the toxic substance responsible for kernicterus, but this is not a
certainty. Very high bilirubin levels (in the 30 mg% range) most often do not result in
kernicterus if no hemolytic disease is present. However, bilirubins in the 20 mg%
range due to Rh incompatibility or G6PD deficiency, often result in kernicterus. The
paradox that a very high bilirubin due to non-hemolytic causes has a lower kernicterus
risk while a moderately high bilirubin due to Rh incompatibility or G6PD deficiency
has a higher kernicterus risk, suggests that bilirubin itself may not be the direct cause
of kernicterus. Bilirubin may only be a marker of the true toxic substance that causes
kernicterus. This phenomenon may explain why the risk of kernicterus is not
determined by bilirubin levels alone.
Jaundice can be detected clinically with tactile blanching of the skin revealing an
underlying yellow color. The examination should be done in a well-lit, neutral light.
Jaundice usually begins on the face and progresses caudally. The presence of scleral
icterus should be assessed. Generally, the farther the jaundice progresses down the
body, the higher the total serum bilirubin (3). The more intense the color (which can
approach a yellow-orange) also suggests a higher total serum bilirubin. Jaundice may
be clinically detected with a total serum bilirubin of 5 mg%. The presence of jaundice
in particularly dark skinned newborns can be difficult to assess. Any time there is
uncertainty, the recommendation is to check a total serum or transcutaneous bilirubin.
Universal screening has been recently recommended, perhaps simultaneously with the
newborn screen. If a patient is under phototherapy, jaundice is difficult to visually
assess because phototherapy preferentially reduces bilirubin concentrations near the
skin. If assessing a patient under phototherapy, jaundice severity is best determined
by examining unexposed sites (e.g., under the eye shield) and phototherapy should be
interrupted during the exam. If the skin is green or bronze colored, this suggests an
elevated direct (conjugated) bilirubin fraction, so a fractionated bilirubin should be
obtained.
The patient should also be assessed for pallor, plethora and
hepatosplenomegaly.
It is essential to distinguish whether the jaundice is physiologic or pathologic.
Jaundice noted within the first 24 hours is pathologic and a total serum bilirubin
should be drawn. Early jaundice is usually related to hemolysis, infection, drug
effect, neonatal hepatitis or liver enzyme defects (e.g., Crigler-Najjar-deficiency of
UDPGT) (7). A total bilirubin greater than 17 mg% in a full term neonate is
pathologic (2). Jaundice that persists beyond 2 weeks should be evaluated beginning
with a fractionated bilirubin (5). Direct hyperbilirubinemia is also considered
pathologic, (i.e., direct bilirubin >1.5-2 mg% or > 20% of the total bilirubin) (8).
Direct (conjugated) hyperbilirubinemia cases are relatively uncommon. The
differential diagnosis includes neonatal hepatitis, biliary atresia, sepsis, metabolic
disorders (e.g., galactosemia), and hepatotoxicity from hyperalimentation. A detailed
discussion of direct hyperbilirubinemia is beyond the scope of this chapter. One of
the principal diagnoses to exclude is biliary atresia which is associated with dark urine
or light colored stool. Early surgical intervention done prior to 2 months of age
reduces mortality and the probability of future liver transplantation (refer to the
chapter on biliary atresia).
Indirect (unconjugated) hyperbilirubinemia, is more common and presents a risk for
kernicterus. The most common causes of indirect hyperbilirubinemia are physiologic,
breast milk, breast feeding with a large postnatal weight loss (3), ABO
incompatibility, cephalohematoma, bruising, G6PD deficiency, and East Asian
ethnicity. Of these, G6PD deficiency poses a higher risk of kernicterus, but ABO
incompatibility is a more common reason for exchange transfusion.
Rh
incompatibility is less common, but represents a high risk of kernicterus.
Breastfeeding jaundice is related to inadequate intake by the newborn (i.e.,
components of poor feeding and dehydration are contributory). Breast milk jaundice
(i.e., due to breast milk itself) has its onset later than breastfeeding jaundice. It is
related to increased enterohepatic circulation and is relatively uncommon.
Uncommon causes of indirect hyperbilirubinemia include other RBC membrane
defects (e.g., hereditary spherocytosis) and hemoglobinopathies. In the pilot
kernicterus registry, 31% of the cases were idiopathic (no identified cause),
approximately equal to the incidence associated with G6PD deficiency (3).
A rise in bilirubin of greater than 0.5 mg% per hour and failure to control
hyperbilirubinemia despite phototherapy are suggestive of hemolytic disease. ABO
incompatibility occurs in approximately 20% to 25% of pregnancies of which
significant hemolysis occurs in 10%. G6PD deficiency (X-linked recessive) occurs in
African, Mediterranean, and Southeast Asian ethnic groups. In our community, some
pediatricians routinely screen for G6PD in males delivered to mothers of high risk
ethnic groups (e.g., Filipino).
Prenatal testing includes maternal blood typing and screening for antibodies to major
RBC antigens (1). Rh incompatibility occurs with an Rh negative mother (usually not
a primigravida) and an Rh positive baby. The routine use of RhoGAM at 28 weeks
gestation and following delivery or pregnancy termination is generally effective in
preventing maternal Rh sensitization (i.e., the production of anti-Rh antibodies by the
mother). An adequate amount of RhoGAM must be given to neutralize the amount of
Rh Ag or the volume of blood from a feto-maternal transfusion. Thus, the incidence
of Rh isoimmunization and Rh incompatibility is uncommon. The Rh antigen is
markedly more antigenic than the A or B antigen. In situations where the maternal
blood type and antibody screening results are unknown, an Rh and Coombs test
should be performed on the baby's blood (1).
In ABO incompatibility, the mother's blood type is O. More commonly, the babies
blood type is A rather than B. Hemolysis in ABO isoimmunization usually has a
positive direct antiglobulin testing (DAT), also known as a direct Coombs test.
Clinically significant hemolysis is associated with a decreasing hemoglobin,
hematocrit and an elevated reticulocyte count. Due to phagocytic removal of
antibody and portions of the RBC membrane, the smear in ABO incompatibility may
have spherocytes, mimicking spherocytosis. Therefore, a CBC with differential,
reticulocyte count and a smear should be requested with suspected hemolysis.
Nucleated RBCs may also be present with more severe causes of hemolysis, but is
classically associated with Rh incompatibility.
If the baby is at risk for G6PD deficiency or other hemolytic diseases, appropriate
testing should be done. When a G6PD level is obtained, it is important to realize that
false negatives may occur in the face of active hemolysis because G6PD is increased
in nucleated red blood cells.
An evaluation should be done for newborns with feeding intolerance, behavioral
changes, hepatosplenomegaly, excessive weight loss, and instability of vital signs
regardless of clinical detection of jaundice. Urine that is positive for reducing
substances, but negative for glucose is suggestive of galactosemia. Galactosemia, a
cause of direct hyperbilirubinemia, is one of the over 30 metabolic disorders included
in the expanded newborn screen.
Educating parents about proper infant feeding practices (both breast and bottle),
detecting their infant's hydration status, observing changes in behavior, and how to
detect and report worsening jaundice, are extremely important anticipatory guidance
measures. Parents should also be counseled that jaundice is common, but in rare
instances, it can lead to severe morbidity and mortality which is largely preventable.
At follow-up, the primary care physician should document the presence/absence of
jaundice and/or the serum bilirubin level. The pediatrician's goal is to discharge a
functional maternal-infant dyad with early follow up (in 1-2 days) if the infant is
discharged from the hospital at less than 48 hours of age. If certain risk factors exist
such as blood group incompatibility or prematurity, or if early follow-up cannot be
scheduled, discharge should be delayed until after the infant has been monitored for
an appropriate period of time. Some of the patients with kernicterus had a bilirubin of
less than 25 mg% and did not have predictable risk factors (9). Potential separation of
the parent and newborn needs to be minimized and weighed against the risks of
hyperbilirubinemia complications. In this community, most mothers are discharged
within 48 hours of a vaginal delivery and 3-4 days post C-section. Discharging the
mother prior to the newborn can affect bonding and breastfeeding. This needs to be
weighed against the risk for significant hyperbilirubinemia and compliance with
follow up.
The clinician should be responsive to parents who are concerned about their child's
degree of jaundice, feeding frequency or volume, drowsiness, and/or irritability (3).
Caution should be used when sending patients home while awaiting lab results.
Never presume that a lab value is falsely elevated. Parents should also be counseled
to seek medical attention for jaundice that persists beyond 2 weeks of age.
Bhutani developed an hour specific bilirubin nomogram in healthy term and near term
newborns with a negative direct Coombs (12). The patient population consisted of
term and near-term appropriate for gestational age (AGA) newborns. Total serum
bilirubins (TSB) of greater than or equal to 8 mg% at 24 hours, greater than or equal
to 14 mg% at 48 hours, and greater than or equal to 16 mg% at 72 hours were above
the 95th percentile. A TSB of greater than or equal to 17 mg% during the first week
of life was above the 95th percentile. This group is at higher risk for bilirubininduced neurologic dysfunction (BIND), including kernicterus. Bhutani advocates
universal bilirubin screening with early follow up to also catch neonates who may
move up from the lower percentiles.
The 1994 AAP Practice parameter (5) is an appropriate guideline for managing
jaundice in the healthy term newborn without hemolysis. The guideline has agebased recommendations for therapy.
At 25 to 48 hours, phototherapy is
recommended at a bilirubin of greater than or equal to 15 mg%. At 49 to 72 hours,
the threshold increases to 18 mg%. From greater than 72 hours, the threshold is 20
mg%. If G6PD deficiency or other kernicterus risk factors are present, it is advisable
to have a lower threshold to begin phototherapy. The AAP Practice parameter also
contains recommendations for exchange transfusion. Table 1 below describes
hyperbilirubinemia treatment guidelines for preterm infants. Table 2 below describes
hyperbilirubinemia treatment guidelines for term infants.
Table 1 - Suggested Maximum Indirect Serum Bilirubin Concentrations (mg%) in
Preterm Infants (10)
Birthweight (g)
Uncomplicated
Complicated*
<1,000
12-13
10-12
1,000-1,250
12-14
10-12
1,251-1,499
14-16
12-14
1,500-1,999
16-20
15-17
2,000-2,500
20-22
18-20
For table 1 above, phototherapy is usually started at 50% to 70% of the maximum
indirect levels. If values greatly exceed this level, if phototherapy is unsuccessful in
reducing the maximum bilirubin level, or if there are signs of kernicterus, exchange
transfusion is indicated. *Complicated cases include those associated with perinatal
asphyxia, acidosis, hypoxia, hypothermia, hypoalbuminemia, meningitis,
intraventricular hemorrhage, hemolysis, hypoglycemia, or signs of kernicterus (10).
Table 2 - Treatment Strategies for Indirect Hyperbilirubinemia in Healthy Term
Infants Without Hemolysis (10)
Intensive phototherapy
and preparation for
Age
exchange transfusion if
Exchange
(hrs)
Phototherapy
phototherapy fails
transfusion
24-48
49-72
>72
15-18
18-20
20
25
30
30
20
25
25
If the initial bilirubin on presentation is high, intense phototherapy should be initiated
and preparation made for exchange transfusion. If the phototherapy fails to reduce the
bilirubin level to the levels noted on the column to the right (table 2), an exchange
transfusion should be initiated. Intensive phototherapy usually reduces serum
bilirubin levels 1 to 2 mg% in 4 to 6 hours. This is often done with IV fluid
administration at 1 to 1.5 times maintenance and oral alimentation should continue
(10).
Hyperbilirubinemia of the degree noted in the 24-48 hours line of table 2, is unusual
and should suggest hemolysis, concealed hemorrhage, or causes of conjugated (direct)
hyperbilirubinemia (10).
Jaundice suddenly appearing in the second week of life or continuing beyond the
second week of life with significant hyperbilirubinemia levels to warrant therapy
should be investigated in detail, as it most probably is due to a serious underlying
cause such as biliary atresia, galactosemia, hypothyroidism, or neonatal hepatitis (10).
Supplementing feeds with water or dextrose has been associated with higher bilirubin
levels. Use of metalloporphyrins which inhibit bilirubin production has been limited
to study trials in newborns. The mainstay of treatment is phototherapy with a
wavelength of 450 nm. It induces photoisomerization of bilirubin, forming lumirubin
which is water soluble and excreted in the urine. Phototherapy is generally delivered
by fluorescent lights, spot lights, or fiberoptics, the latter generating less heat. Unless
there is a high risk for an exchange transfusion, phototherapy can usually be
discontinued for an hour to allow for neonatal care. Discontinuation of phototherapy
in a term baby without hemolysis generally is not associated with rebound
hyperbilirubinemia, i.e., a significant increase in bilirubin.
Although phototherapy may be commonly thought of to prevent kernicterus, this is
less than accurate, since if the patient is at significant risk of kernicterus, an exchange
transfusion should be done. Phototherapy's major role is to avoid an exchange
transfusion. By implementing phototherapy, the belief is that this will reduce the
likelihood that bilirubin levels will reach these exchange transfusion levels, thus
avoiding an exchange transfusion.
In term neonates with hemolytic disease, if the bilirubin approaches 20 mg% despite
medical management, informed consent should be obtained for an exchange
transfusion. In preterm neonates less than 37 weeks gestation, most neonatologists
will await serum bilirubin levels of 15-20 mg% before considering an exchange (11).
Exchange transfusion removes bilirubin and antibodies causing hemolysis. Twice the
patient's blood volume is exchanged (i.e., double volume exchange) while monitoring
cardiovascular stability. Risk factors include those related to catheters, electrolyte
imbalance and blood products superimposed onto preexisting medical problems.
Thrombocytopenia is common. Necrotizing enterocolitis, graft versus host disease,
and death have also been reported as complications of exchange transfusion.
Does phototherapy reduce the need for an exchange transfusion? In ABO
incompatibility, prophylactic phototherapy was not shown to be superior to no therapy
at all in this regard (13). However, phototherapy intensities have increased since the
time of that study and anecdotally, it appears that fewer exchange transfusions are
being done, possibly due to more routine use of phototherapy. Additionally,
phototherapy appears to be harmless, so its utilization is reasonable to treat
moderately high bilirubin levels.
Although extreme bilirubin levels are associated with kernicterus, the adverse effects
of moderate hyperbilirubinemia may be more difficult to identify. For example,
subclinical adverse effects, learning disabilities or behavioral disorders would be
more difficult to causally link to moderate hyperbilirubinemia, since this would only
be manifested in later childhood.
Most cases of hyperbilirubinemia resolve without sequelae. However, there may be
impairment of auditory nerve conduction (2). After significant hyperbilirubinemia,
the patient should undergo auditory brainstem response testing (6).
Questions
1. Which of the following factors leads to neonatal hyperbilirubinemia?
a. Shortened neonatal red cell life span.
b. Impaired excretion of unconjugated bilirubin.
c. Limited conjugation of bilirubin in the liver.
d. Increased enterohepatic circulation.
e. All of the above.
2. True/False: Hemoglobin degradation results in the formation of biliverdin and
carbon monoxide.
3. A total serum bilirubin >17 mg% in a term neonate is:
a. physiologic
b. pathologic
4. In G6PD deficiency, there is hyperbilirubinemia on the basis of:
a. hemolysis
b. decreased conjugation
c. both
d. neither
5. True/False: In Asians, a variant in UDPGT is associated with neonatal
hyperbilirubinemia.
6. True/False: Systemic sulfonamide medications are avoided in the newborn
because they displace bilirubin from albumin and increase free bilirubin.
7. True/False: Breast milk jaundice is more common than breast feeding jaundice.
8. True/False: Supplementation of breast feeding with water or dextrose lowers the
serum bilirubin.
9. True/False: Discontinuation of phototherapy in a healthy, term neonate is usually
associated with rebound hyperbilirubinemia.
10. Which of the following factors should be strongly considered in determining
whether an exchange transfusion is indicated in a term neonate with an indirect
bilirubin of 21 mg%.
a. Age of the neonate (time since birth).
b. Whether the cause is hemolytic or non-hemolytic.
c. The presence of other clinical factors such as intraventricular hemorrhage
or meningitis.
d. All of the above.
e. None of the above.
Answers to questions
1.e, 2.True, 3.b, 4.c, 5.True, 6.True, 7.False, 8.False, 9.False, 10.d
References
1. Nazarian LF. Personal Reflections on the AAP Practice Parameter on
Management of Hyperbilirubinemia in the Healthy Term Newborn. Pediatr Rev
1998;19(3):75-77.
2. Dennery PA, Seidman DS, Stevenson DK. Neonatal Hyperbilirubinemia.
N Engl J Med 2001;344(8):581-590.
3. Johnson LH, Bhutani VK, Brown AK. System-based Approach to
Management of Neonatal Jaundice and Prevention of Kernicterus. J Pediatr
2002;140(4):396-403.
4. JCAHO Sentinel Event Alert: Kernicterus Threatens Healthy Newborns.
JCAHO
website,
2001.
http://www.jcaho.org/about+us/news+letters/sentinel+event+alert/sea_18.htm
5. American Academy of Pediatrics. Practice Parameter: Management of
Hyperbilirubinemia in the Healthy Term Newborn. Pediatrics 1994;94(4):558-565.
6. AAP Subcommittee on Neonatal Hyperbilirubinemia: Neonatal Jaundice
and Kernicterus. Pediatrics 2001;108(3):763-765.
7. Jain SK. Index of suspicion. Case 3. Diagnosis: jaundice. Pediatr Rev
2001;22(8):271-276.
8. Bisgard L. Visual diagnosis: a 10-week-old infant who has jaundice.
Pediatr Rev 2001;22(12):408-412.
9.
Poland RL.
Preventing kernicterus: almost there.
J Pediatr
2002;140(4):385-386.
10. Stoll BJ, Kliegman RM. Chapter 98.3. Jaundice and Hyperbilirubinemia
in the Newborn. In: Behrman RE, Kliegman RM, Jenson HB (eds.). Nelson
Textbook of Pediatrics, 16th edition. 2000, Philadelphia: WB Saunders, pp. 513-517.
11. Chapter 8. Neonatal Complications. In: American Academy of
Pediatrics and American College of Obstetricians and Gynecologists. Guidelines for
Perinatal Care, 5th edition. 2002, Elk Grove Village, IL: AAP, pp. 239-240.
12. Bhutani VK. Predictive Ability of a Predischarge Hour-specific Serum
Bilirubin for Subsequent Significant Hyperbilirubinemia in Healthy Term and Near
Term Newborns. Pediatrics 1999;103(1):6-14
13. Maurer HM, Kirkpatrick BV, McWilliams NB, et al. Phototherapy for
Hyperbilirubinemia of Hemolytic Disease of the Newborn. Pediatrics 1985;75(2Supp):407-412.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
Respiratory distress:
A 2 hour old, 38 week gestation, 3 kg male infant was born to a 25 year old G1P1 A+,
VDRL negative, Hepatitis B negative, GBS unscreened, Rubella immune, woman
who had an uncomplicated pregnancy, labor and delivery. Apgar scores were 9/9. He
was sent to the newborn nursery. He breast fed 1 hour ago without concerns. He now
presents with respiratory distress.
Exam: VS T 37, HR 160, RR 80, BP 60/35 (mean 45), oxygen saturation 95% in 2
L/min oxygen via mask. Wt 3kg (50%), Lt 48cm (50%), HC 33.5 cm (50%). He is a
term male with obvious respiratory distress and tachypnea. Skin is pale and pink
without petechiae, ecchymoses, or lesions. His head is normocephalic with molding.
His anterior fontanel is flat. His face is symmetrical with normal palpebral fissures,
normal red reflexes, patent nares, normal ears, no clefts, and no neck masses. His
chest is symmetric with equal and clear breath sounds. Mild to moderate chest
retractions are present. Heart is regular with a normal S1, split S2, and no murmurs.
His pulses are normal. His abdomen is soft and round with normal bowel sounds, no
masses and no organomegaly. He has normal male genitalia with descended testes.
His hips and anus are normal. He has mild hypotonia.
Laboratory results: CBC: WBC 15,000, 8% bands, 50% segs, 40% lymphs, 2%
monos, Hct 55%, Plt 250,000. No toxic granulations or vacuoles of the neutrophils
are noted. ABG: pH 7.35, PCO2 55 torr, PO2 70 torr, BE -7 in a 30% oxygen hood.
CXR: 10 ribs of inflation, streaky linear perihilar densities, and small scattered
patchy densities bilaterally.
Over the next several hours, the infant develops progressively more distress and a
greater oxygen requirement. He is sent to the newborn special care nursery with
worsening tachypnea (RR 90), more retractions and grunting. He is now in 50% O2
by hood.
What is your differential diagnoses? How would you manage this infant?
This chapter will cover the common problems which cause respiratory distress in the
newborn within the first week of life. Based on the clinical presentation, onset and
gestational age, the most likely diagnosis can be determined.
Respiratory distress is one of the most common presenting problems of newborns.
The constellation of signs and symptoms can be the result of pulmonary, cardiac,
metabolic, infectious, renal, gastroenterological and neurologic pathologic processes.
Newborns with disorders involving any one of these organ systems may present with
varying degrees of tachypnea, retractions, grunting, cyanosis, lethargy and
tachycardia. Given the similar presentations, the circumstances of the newborn's birth
provide important clues to the diagnosis.
Transient tachypnea of the newborn (TTN) is the most common respiratory disorder
of the newborn. These infants are usually full term or slightly preterm. They are not
at risk for other illnesses. Some infants are delivered by cesarean section; some
without labor. The most significant discriminatory findings are the onset of the illness
and the degree of distress exhibited by the infant. Typically, the infant becomes
tachypneic immediately after birth and has mild respiratory distress. They are
neurologically normal. If followed closely, infants remain stable for several hours
and/or begin to improve. The chest radiographs reveal hyperinflation with clear lung
parenchyma except for perihilar linear densities and fluid in the fissures. There
should be no areas of consolidation . The pathophysiological mechanism is the
delayed resorption of fetal lung fluid which eventually clears over the next several
hours to days. A worsening clinical picture should suggest another diagnosis.
Treatment is generally close observation and symptomatic care. Low flow
supplemental oxygen may be necessary for several hours.
Meconium in the amniotic fluid occurs in approximately 20% of pregnancies. As a
consequence, meconium aspiration is considered to be a relatively common event.
Other substances such as blood or amniotic fluid can also be aspirated. Infants with
this disorder typically have symptoms similar to infants with TTN, but the
presentation may suggest a more severe condition. In addition, while many infants
have the onset of symptoms at birth, some infants have an asymptomatic period of
several hours before respiratory distress becomes apparent. Infants with aspiration
syndromes may require more oxygen, and have greater degrees of tachypnea,
retractions and lethargy. The arterial blood gases may reveal more acidosis,
hypercapnia and hypoxemia than in infants with TTN. The chest radiographs vary
between that of TTN with hyperinflation and perihilar infiltrates to significant
heterogeneous lung disease with hyperinflated and hypoinflated areas, patchy and
linear infiltrates and atelectasis.
The pathophysiologic mechanism is the obstruction of large and small airways with
the aspirated material (meconium, blood, amniotic fluid contents). Pulmonary
hypertension may be develop when meconium aspiration occurs in conjunction with
varying degrees of in utero asphyxia. Pulmonary hypertension, which often results
from hypertrophic pulmonary vascular muscular tissue, is a severe condition
characterized by cyanosis from right to left shunting across the atrial septum and
patent ductus arteriosus. As the disease process progresses, the symptoms and
severity of hypoxemia increase over the subsequent hours. While TTN is in the
differential diagnoses initially, this progression should alert the clinician to another
diagnosis such as an aspiration syndrome, pulmonary hypertension or infection.
Treatment may include supplemental oxygen, mechanical ventilation and specific
treatment for pulmonary hypertension, which includes high supplemental oxygen,
high frequency mechanical ventilation, inhaled nitric oxide therapy and in the most
severe cases, extracorporeal membrane oxygenation therapy (ECMO).
The duration of distress with mild to moderate aspiration syndromes is from several
hours to days. Aspiration can occur in utero or during the intrapartum period as well
as during the early postpartum period. Since meconium aspiration is the most
common problem, much effort has been made over the last 30 years to prevent this
disease by reducing intrapartum and postpartum aspiration. Thorough suctioning of
the oropharynx with a large bore catheter upon the delivery of the head is typically
performed by the obstetrician. The pediatrician, needs to assess the quality of the
meconium (thin, moderate or thick) and the state of the newborn before determining
what is needed after birth. A large randomized trial has confirmed that aggressive
intubation is not necessary for most infants with meconium in the amniotic fluid. The
recommendations provided by the NRP (Neonatal Resuscitation Program) are to
suction the oropharynx of all infants after the delivery of the head and to intubate and
suction the trachea of any infant with meconium in the amniotic fluid, if the infant is
depressed (weak. respiratory effort, hypotonia and/or bradycardia).
The sudden onset of significant respiratory distress should raise the possibility of an
air leak syndrome. The most common air leak syndromes are pneumomediastinum,
pneumothorax and pneumopericardium. In addition to respiratory distress, a severe
air leak condition may cause hypotension (due to decreases in cardiac output),
muffled heart tones, abdominal distention, asymmetric chest shape and deviation of
the cardiac sounds. Chest radiographs are diagnostic with free air in the hemithorax
and a visible edge of the collapsed lung. If under tension (i.e., a tension
pneumothorax), clinical deterioration will be rapid, the mediastinum will be deviated
to the opposite (contralateral) side and the ipsilateral diaphragm will be depressed.
The elevation of the thymus with a sail or bat wing sign suggests a
pneumomediastinum.
The heart is outlined with a halo of air in a
pneumopericardium. Hypotension and bradycardia occur rapidly in a tension
pneumothorax or pneumopericardium (cardiac dysfunction is due to reduced venous
return due to compression of the heart and mediastinal vascular structures). The air
leak syndrome known as pulmonary interstitial emphysema (PIE) is usually observed
as a consequence of mechanical ventilation in an infant with severe respiratory
distress syndrome.
Treatment of significant air leak syndromes requires immediate air evacuation
(thoracentesis or pericardiocentesis) with a needle or small catheter, followed by chest
or pericardial tube insertion. Pneumomediastinum does not require drainage. In cases
other than a bronchopleural fistula, the air leak will usually seal within a few days.
Respiratory distress syndrome (RDS) is the most common disorder of the premature
infant. Most infants are less than 34 weeks gestation and the incidence and severity
increase with decreasing gestation age. These premature infants have progressively
more severe respiratory distress after birth. The classic findings of cyanosis, grunting,
nasal flaring, intercostal and subcostal retractions and tachypnea are present. The
chest radiograph reveals decreased lung inflation with diffuse symmetrical
reticulogranular (ground glass appearance) lung fields and air bronchograms. Oxygen
requirements progressively increase over the first few hours after birth. The presence
of apnea suggests severe disease accompanied by refractory hypoxemia and acidosis.
While the lung's structural immaturity contributes to the pulmonary dysfunction, the
major reason for this disorder is surfactant deficiency. Without surfactant, the surface
tension of the alveolar sacs is high, leading to an increased tendency of the alveoli to
collapse. Laplace's law describes the behavior of the alveoli without surfactant. This
relationship states that as the radius of the air filled alveolus decreases, the pressure
within the alveolus increases. This increased pressure requires an equivalent external
opposing pressure to keep the alveolus inflated. Without the opposing pressure, the
gas under this pressure is forced out of the alveolus. If the alveolus is connected to an
adjacent alveolus with a larger radius, air will preferentially inflate the larger alveolus
and ultimately collapse the smaller alveolus. This leads to the network of air-filled
alveoli juxtaposed to atelectatic alveoli and creates the reticulogranular pattern
(ground glass appearance) of the lung. The air bronchograms are created by
atelectatic alveoli outlining the adjacent rigidly distended airways. Air filled right and
left mainstem bronchi are not visible if they are superimposed over air filled lungs,
but when they are superimposed over partially collapsed, fluid filled lungs (as in
RDS), the air filled bronchi are visible as air bronchograms. Grunting is the infant's
attempt to maintain the pressures and gas volume within the lung by causing
expiratory braking using the vocal cords (the glottis is partially closed during
exhalation to maintain alveolar distending pressure during exhalation). Surfactant
reverses this process. The phospholipids and surfactant related proteins, contained in
surfactant, spread along the air liquid interface to decrease alveolar surface tension.
Therefore the pressure required to keep the alveoli inflated is lower. Furthermore, the
surfactant molecules contribute to the larger alveoli developing a higher surface
tension during inspiration and a lower surface tension (as the alveoli deflate) during
expiration when the surfactant molecules become more compact along the air liquid
interface.
The treatment of RDS generally includes positive pressure ventilation and artificial
surfactant replacement. The first purely synthetic surfactant is no longer available.
Today, several types of animal based surfactants have been approved for clinical use.
After endotracheal intubation, surfactant suspension is administered through the
endotracheal (ET) tube and the infant is supported, until extubation is possible, with
positive pressure ventilation and continuous positive airway pressure (CPAP) therapy
as needed. High frequency ventilation has been shown to improve the short term
management of these infants.
Moderately premature infants (29 to 34 weeks gestation) are usually extubated within
several days after treatment. However, extremely premature infants (23 to 28 weeks
gestation) may continue to require positive pressure respiratory support for several
weeks. They are at high risk for bronchopulmonary dysplasia or chronic respiratory
insufficiency of the premature. Bronchopulmonary dysplasia is the chronic lung
disease consequence of early acute lung disease and/or lung immaturity of the
premature infant. Chronic respiratory insufficiency of the premature develops despite
early improvement after surfactant therapy and mechanical ventilation.
Infectious pneumonia in newborns is relatively rare. However, premature infants
have at least a 10 fold increased incidence of infections when compared to term
infants. Mothers with intrapartum fever and prolonged rupture of membranes (>1824 hours) have a greater risk of transmitting infections to their infants. This risk can
be reduced by administering intrapartum antibiotics for mothers with high risk
pregnancies or women who are group B Streptococcus (GBS) carriers. However, the
use of ampicillin for the GBS infections has increased the incidence of ampicillin
resistant coliform infections. The most common bacterial organisms which infect
neonates are the GBS, coliforms (E. coli being the most common member of this
group), and Listeria monocytogenes. Infected infants present either immediately after
birth with respiratory distress or they may present after several hours of an
asymptomatic period. The degree of respiratory distress initially may mimic any
respiratory disorder. Infants may have fevers or become hypothermic. The symptoms
progressively increase in severity and if not treated may lead to shock, DIC and death.
Due to the serious consequences associated with delays in treatment for infections,
many infants with non-infectious conditions are evaluated and empirically treated
with antibiotics for this possibility. The chest radiographs may resemble RDS (with
reticulogranular infiltrates and air bronchograms), TTN or aspiration syndromes (with
linear or patchy densities). It is unusual to have lobar consolidation from infection in
the newborn. A term infant with RDS should be considered to have pneumonia until
proven otherwise. The CBC may reveal either a leukocytosis or leukopenia. A left
shift with greater than 20% band forms of the total neutrophils is suggestive of
infection as are neutrophilic vacuoles and toxic granulation. Platelet counts may be
normal or decreased. In severe cases, a coagulopathy with elevated PT and PTT and
depressed fibrinogen levels may be present. The gold standard still remains the blood
culture or culture of lung and tracheal secretions. Treatment with an aminoglycoside
and penicillin is standard to treat for the common organisms. Supportive care may
include mechanical ventilation, supplemental oxygen, inotropic agents for
hypotension and nitric oxide for infection associated pulmonary hypertension. The
mortality from infections has decreased from 50% to 20% with more aggressive
intensive care.
Some congenital malformations of the cardiopulmonary system will be addressed
here. The first is the infant with cyanotic heart disease. Most infants with
transposition of the great arteries, tetralogy of Fallot and hypoplastic right and left
heart syndromes, will present in the newborn period. Most infants with cyanotic heart
disease typically have a paucity of respiratory distress symptoms except for cyanosis
or duskiness. A murmur is usually present, but may be absent. Typically the chest
radiograph reveals a normal sized heart or cardiomegaly with clear lung fields and
decreased vascular markings (due to diminished pulmonary blood flow). When the
infant develops respiratory symptoms, it is usually from severe hypoxemia or
acidosis. The infant who is cyanotic with respiratory distress and does not respond to
supplemental oxygen (i.e., their oxygen saturation does not improve significantly
when given supplemental oxygen). Infants with both cyanosis and respiratory distress
may have chest radiographs typical of pulmonary disease. The hyperoxy test
(measuring the arterial pO2 while the infant is breathing 100% oxygen) is helpful in
distinguishing cyanotic heart disease from severe respiratory disease.
The
echocardiogram is also diagnostic and will distinguish between cyanotic heart disease
and persistent pulmonary hypertension.
Therapy for cyanotic heart disease consists of medical support until definitive surgical
repair can take place. In many instances, patency of the ductus arteriosus is necessary
to maintain mixing of pulmonary and systemic circulations. This is accomplished
with an intravenous infusion of prostaglandin E1 infusions. Later, an artificial shunt
is created from the aorta to the pulmonary arteries.
Moderate oxygen
supplementation to keep oxygen saturations approximately 80% or higher and mild
acidosis to maintain a fetal type circulation is attempted to preserve pulmonary
function. Multistaged open heart surgery may be necessary for most complex
cyanotic heart diseases.
Structural abnormalities of the pulmonary system may also cause respiratory distress.
Infants with congenital diaphragmatic hernia frequently present in the immediate
newborn period with respiratory distress and refractory cyanosis. The abdomen is
scaphoid since the intestines are in the thorax. Bowel sounds are heard over the chest
if air enters the intestines from spontaneous breathing or mask valve ventilation. The
chest radiograph reveals a bowel gas pattern typically in the left hemithorax with a
mediastinal shift to the right. The heart compresses the right lung which may also be
hypoinflated or hypoplastic. Surgery to remove the bowel from the thorax and close
the diaphragmatic defect is necessary after the infant has been stabilized. High
frequency ventilation and nitric oxide therapy are used to treat the bilateral
hypoplastic lungs.
The hypoplastic lungs develop excessive and abnormal
musculature of the pulmonary vessels which lead to pulmonary hypertension. In the
most severe cases, extracorporeal membrane oxygenation (ECMO) therapy is used to
support the cardiopulmonary failure. However, despite aggressive treatment,
approximately 50% of the infants with this condition do not survive. Lung volumes
may reach normal values, but there is a persistence of decreased number of alveoli
(emphysema). Please refer to the focused chapter on congenital diaphragmatic hernia.
In summary, the term infant with respiratory distress usually has transient tachypnea
of the newborn. However, based on the time of onset and the progression and
severity of the symptoms, other causes of respiratory distress must be entertained.
Premature infants usually have RDS, but must be considered to be at risk for
infection. In the case presentation at the beginning of this chapter, the later onset of
respiratory distress which increases in severity with time, suggests either aspiration or
an infectious process. The unremarkable CBC makes a pneumonia less likely and
possibly supports an aspiration syndrome; however the CBC may change with time.
Empiric antibiotic therapy is indicated. Management is supportive and supplemental
oxygen should be continued. A repeat arterial blood gas is indicated and if the pCO2
is elevate, then consider mechanical ventilation. If the chest radiographs suggest
significant atelectasis or a further increase in FiO2 is required, either nasal CPAP
(continuous positive airway pressure) or mechanical ventilation with positive
pressures may enhance oxygenation.
Simplified summary of some of the major newborn respiratory conditions:
RDS:
Clinical factors: Prematurity
CXR: Ground glass appearance
TTN:
Clinical factors: Short labor, C-section delivery
CXR: Fluid in the fissures, central/perihilar congestion
Meconium aspiration:
Clinical factors: Meconium in amniotic fluid
CXR: Infiltrates
Pneumonia:
Clinical factors:
Prolonged rupture of membranes, maternal GBS,
prematurity.
CXR: Infiltrates or hazy lungs (may be identical to RDS).
Pneumothorax:
Clinical factors: Sudden deterioration, often while on positive pressure
ventilation.
CXR: Collapsed lung, free air in the hemithorax.
Cyanotic congenital heart disease:
Clinical factors: Heart murmur, persistent hypoxia despite supplemental
oxygen.
CXR: Hypoperfused lungs (lungs appear darker). CXR is often normal.
Questions
1. What is the most common cause of respiratory distress in newborns?
2. When is the onset of symptoms for transient tachypnea of the newborn and
how might this help distinguish TTN from other disorders?
3. Aspiration syndromes can be caused by what types of materials?
4. The sudden onset of significant respiratory distress and hypotension should
suggest what respiratory disorder?
5. Respiratory distress syndrome of the premature infant is caused by what
deficiency? What is the radiographic manifestation of this deficiency?
6. What organisms commonly cause newborn pneumonia?
7. What disorder would you consider in a cyanotic infant without respiratory
distress?
Answers to questions
1. TTN
2. TTN symptoms occur soon after birth. Later onset of symptoms should
suggest other disorders.
3. Meconium, blood, amniotic fluid.
4. Air leaks such as a tension pneumothorax.
5. Surfactant deficiency, which causes some alveoli to collapse next to alveoli
which are emphysematous. Some atelectatic alveoli are adjacent to rigid bronchi.
These conditions lead to a reticulogranular infiltrate (ground glass) and air
bronchogram pattern on the chest radiograph.
6. Group B Streptococcus, gram negative rod organisms (usually E. coli) and
Listeria monocytogenes.
7. Cyanotic congenital heart disease.
References
1. Hansen T, Corbet A. Pulmonary physiology of the newborn. In: Taeusch
WH, Ballard RA (eds). Avery's Diseases of the Newborn, 7th edition. 1998,
Philadelphia: WB Saunders Company, pp. 562-575.
2. Gomez M, Hansen T, Corbet A. Principles of Respiratory Monitoring and
Therapy. In: Taeusch WH, Ballard RA (eds). Avery's Diseases of the Newborn, 7th
edition. 1998, Philadelphia: WB Saunders Company, pp. 585-590.
3. Hansen T, Corbet A. Disorders of the transition. In: Taeusch WH, Ballard
RA (eds). Avery's Diseases of the Newborn, 7th edition. 1998, Philadelphia: WB
Saunders Company, pp. 602-630.
4. Hansen T, Corbet A. Air block syndromes. In: Taeusch WH, Ballard RA
(eds). Avery's Diseases of the Newborn, 7th edition. 1998, Philadelphia: WB
Saunders Company, pp. 631-633.
5. Hansen T, Corbet A. Neonatal pneumonias. In: Taeusch WH, Ballard
RA, editors. Avery's Diseases of the Newborn. 7th ed. Philadelphia: WB Saunders
Company; 1998. p. 648-57.
6. Hansen T, Corbet A, Ballard RA. Disorders of the chest wall and
diaphragm. In: Taeusch WH, Ballard RA (eds). Avery's Diseases of the Newborn,
7th edition. 1998, Philadelphia: WB Saunders Company, pp. 685- 692.
7. Long WA, Frantz EG, Henry W, et al. Evaluation of newborns with
possible cardiac problems. In: Taeusch WH, Ballard RA (eds). Avery's Diseases of
the Newborn, 7th edition. 1998, Philadelphia: WB Saunders Company, pp. 711-734.
8. Soll BJ, Kliegman RM. Respiratory tract disorders. In: Behrman RE,
Kliegman RM, Jenson HB (eds). Nelson Textbook of Pediatrics, 16th edition. 2000,
Philadelphia: WB Saunders Company, pp. 496-510.
9. Hartman GE. Diaphragmatic hernia. In: Behrman RE, Kliegman RM,
Jenson HB (eds). Nelson Textbook of Pediatrics, 16th edition. 2000, Philadelphia:
WB Saunders Company, pp. 1231-1233.
10. Initial steps in resuscitation. In: Kattwinkel J (ed). Neonatal
Resuscitation Textbook, 4th edition. 2000, Elk Grove Village: American Academy
of Pediatrics/American Heart Association, pp. 2.3- 2.9.
11. Possmayer F. Physicochemical aspects of pulmonary surfactant. In:
Polin RA, Fox WW (eds). Fetal and Neonatal Physiology, 2nd edition. 1998,
Philadelphia: WB Saunders Company, pp.1263-1265.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
Meninigitis
Case 1
A six month old male presents to the emergency department with a history of
lethargy. He was seen 3 days ago with fever and URI symptoms, diagnosed with
otitis media and treated with oral amoxicillin. This morning he had become irritable
and was less active than usual. He has vomited three times and his urine output is
noticeably decreased. He has no diarrhea.
Exam: VS T 40.0, P 90, R 30 (irregular), BP 120/90, weight 8kg. He is lethargic and
arousable only to painful stimuli. His anterior fontanel is full and tense, and he has
questionable neck rigidity. His TMs are red and bulging. His pupils are reactive, but
his eyes do not focus well on his parents. His heart, lungs and abdomen are normal.
His color and perfusion are good. He has no petechiae. He moves all his extremities
weakly and his DTRs are hyperactive.
A CBC, blood culture and chemistry panel are drawn. An IV is started. Since an
increased ICP (intracranial pressure) is suspected, a lumbar puncture (LP) is initially
delayed and he is immediately given 500 mg of ceftriaxone IV. A stat CT scan of the
brain is normal, so an LP is done and the CSF (cerebrospinal fluid) is visibly hazy.
An infectious disease consultant is called to inquire about IV dexamethasone and
vancomycin. Both are recommended and given. The CSF results return 1 hour later
showing 450 WBCs, 95% segs, 5% monos, total protein 75, glucose 25 mg/dl. Gram
stain of the CSF shows many WBCs with few gram positive cocci. He is admitted to
the pediatric ICU.
The clinical presentation of this patient, with a very rapidly evolving febrile illness,
changes in sensorium and evidence of increased intracranial pressure, is very
compatible with a diagnosis of CNS (central nervous system) infection. Bacterial
meningitis occurs more frequently between the ages of 2 months and two years.
Acquisition of infected aerosolized particles, with initial colonization of the
nasopharynx, is followed by subsequent replication in the regional lymph nodes,
invasion, septicemia and CNS infection. The lack of anticapsular antibodies increases
the risk of invasion. Rarely, the infection is due to spread from a contiguous focus
such as the sinuses, the middle ear, or the mastoids. Bacterial meningitis secondary to
otitis media is an uncommon phenomenon, but when it does occur, it is usually
septicemic in origin, rather than due to direct extension.
Manifestations of bacterial meningitis are variable and depend upon the child's age
and the duration of illness. In young infants, evidence of meningeal inflammation
may be minimal and only irritability and poor feeding may be present. Body
movements of infants with meningitis result in pain, accordingly a strong suspicion of
CNS infection is aroused when the child does not wish to be handled but prefers to
remain motionless. Such paradoxical irritability (which worsens when the child is
carried, rocked or gently bounced), is highly suggestive of meningitis.
The older child will present with more clinical findings, such as nuchal rigidity,
vomiting, lethargy and photophobia. Most cases of meningitis will be either of
bacterial or viral etiology. Common bacterial causes in this age group include
Streptococcus
pneumonia
(pneumococcus)
and
Neisseria
meningitidis
(meningococcus). The advent of a very efficacious vaccine against H. influenzae type
B, has resulted in an almost complete disappearance of meningitis due to this
etiological agent. Prior to this vaccine, this was the most common cause of bacterial
meningitis.
An LP is indicated in patients with clinical findings compatible with meningitis.
Strong consideration should be given to delaying the LP in patients with clinical
findings of increased intracranial pressure. However, antibiotic administration should
not be delayed by this. Antibiotics must still be given immediately once bacterial
meningitis is suspected. The patient in our case has evidence of increased intracranial
pressure since he has decreased sensorium, a bulging tense fontanel, hyperactive
reflexes and changes in the vital signs such as a decreased pulse rate, hypertension
and irregular respirations (Cushing's triad). Accordingly, caution should be taken
before performing the LP due to the possibility of precipitating herniation. A CT scan
of the head is a very rapid and accurate means to confirm increased intracranial
pressure and if present, measures such as the administration of mannitol and
hyperventilation, after rapid sequence intubation, should be instituted before the LP is
done. Analysis of the CSF is usually very useful in confirming the diagnosis of
bacterial meningitis. Acute bacterial meningitis is characterized by an elevated CSF
white count with a predominance of polymorphonuclear cells (neutrophils), a
decreased CSF glucose level, an increased protein value and a positive gram strain
and culture. Administration of oral antibiotics prior to the LP, do not greatly modify
the LP results, except for a slight decrease in the rate of identifying the organism on
gram strain and cultures.
The treatment of meningitis is directed at reducing the damage produced by the
inflammatory response by maintaining adequate cerebral perfusion with the use of
adequate amounts of intravenous fluids and agents that reduce intracranial pressure
and by treating the infection. The use of a third generation cephalosporin such as
cefotaxime (50 mg/kg dose every 6 hours) or ceftriaxone (100 mg/kg day in one dose)
provides coverage for most of the agents responsible (pneumococcus, meningococcus,
H. influenzae type B) for meningitis except for penicillin-resistant pneumococcus
which require the addition of vancomycin. Antibiotics used to treat meningitis must
reliably penetrate the blood brain barrier in addition to reliably cover the organisms
involved. The duration of treatment is dictated mostly by the clinical course but
usually is 5 to 7 days for meningococcal infections and 10 days for infections due to
pneumococcus. Neonatal meningitis has a different group of etiologic bacteria and
antibiotics which are covered in the chapter on neonatal sepsis.
The survival of patients with bacterial meningitis has improved but it still remains a
disease with high morbidity. Approximately half of those with S. pneumoniae and 15
percent of those with H. influenzae meningitis will develop neurological sequela.
Cerebral infarction occurs in 5 to 20 percent of the patients as a result of localized
inadequate perfusion due to local thrombosis, arteriolar vasculitis and phlebitis
secondary to the inflammatory response. Sensorineural hearing loss is the most
common sequela occurring in approximately 15 percent of cases. The hearing loss is
usually severe, bilateral and permanent, and it occurs during the first few days of the
infection. Penetration of bacteria through the internal auditory canal results in
inflammation and destruction of the auditory nerve. Reduction in the incidence of
hearing loss was reported with the use of corticosteroids (dexamethasone) for cases of
H. influenzae meningitis, but proof supporting the benefit of corticosteroids with
other causes of bacterial meningitis is not as evident. The use of corticosteroids is
currently controversial due to the decrease in cases of H. influenzae meningitis (due to
routine H. influenzae vaccine) and the fact that most cases of bacterial meningitis are
now caused by pneumococcus and meningococcus, for which the benefit of
corticosteroids is less proven.
Primary prevention of meningitis is accomplished by the administration of H.
influenzae type B and S. pneumoniae vaccines to infants. Meningococcal vaccine is
also available, but it is not routinely recommended, except adolescents and adults
residing in dormitories or military barracks. Secondary prevention, with antibiotics
such as rifampin is recommended for close contacts of patients with invasive H.
influenzae type B and N. meningitis disease (but not for pneumococcal meningitis).
Case 2
A three year old female presents to the emergency department with a two day history
of headache, nausea, vomiting and fever. She was seen by a physician two days ago
who diagnosed otitis media and prescribed amoxicillin. She has taken six doses. Her
immunizations are up to date. She is conscious, alert and complains of pain over the
neck area. On examination she has pain on flexion of the neck. An LP showed 453
WBCs with 75% neutrophils and a glucose of 50 mg% (blood glucose 90 mg%) and a
protein value of 55 mg%. A gram strain is negative for bacteria. Her headache
improves and she appears less ill following the LP. She is admitted to the hospital
with the diagnosis of viral meningitis. A repeat LP done 20 hours after the initial LP,
shows 315 WBCs with 83% lymphocytes and a glucose value of 75 mg%, blood
glucose of 89 mg%, and protein of 30 mg%. She is largely asymptomatic following
the second LP. CSF cultures remain negative. A CSF PCR for enterovirus is
positive.
Aseptic meningitis is characterized by lymphocytic/monocytic predominance of the
CSF differential. The CSF protein is not as high and the glucose is not as low,
compared to bacterial meningitis. Aseptic meningitis is almost always due to viral
etiologies; however the rare case of tuberculous and fungal meningitis will present as
an aseptic meningitis as well. Patients with viral meningitis can have all of the signs
and symptoms of patients with bacterial meningitis; however, their findings are less
severe. The classic patient with bacterial meningitis is toxic in appearance, irritable,
and/or lethargic, possibly with other signs of sepsis. The typical patient with viral
meningitis is alert and cooperative, but uncomfortable and mildly ill. Young infants
are the most difficult to assess. Older cooperative children who can speak and
express their symptoms are easier to evaluate. A lumbar puncture has two advantages
in cases of viral meningitis in that it will usually ascertain a firm diagnosis and it will
usually provide some degree of headache relief.
CSF neutrophil predominance can be initially seen in up to two thirds of cases of
meningitis due to enterovirus and a slight decrease in the CSF blood glucose ratio
occurs in one fourth of pediatric enteroviral meningitis. The low protein value and
the relative low WBC are also indicative of a viral etiology. Enteroviruses are the
leading cause of aseptic meningitis and account for 90 percent of all cases in which a
pathogen is identified. Infants and children are most commonly affected and the
prognosis is generally excellent. A CSF PCR for enterovirus is highly accurate in
making an etiological diagnosis and will be positive in the great majority of cases.
The repeated LP done 12 to 24 hours after the first will show a rapid shift in the CSF
differential count from neutrophils to mononuclear predominance.
Questions
1. A three year old male presents with a bad headache, nausea, photophobia
and fever (temp 38 degrees). His immunizations are up to date. He is not toxic in
appearance. He is alert and cooperative. He has mild photophobia and mild nuchal
discomfort without rigidity. He can speak and ambulate normally. The remainder of
his exam is unremarkable. If this patient has meningitis, does he/she have bacterial or
viral meningitis? What factors suggest one or the other?
2. An LP is done on the patient in question #1. The results show the
following: 3 RBCs, 200 WBCs, 70% segs, 10% lymphs, 20 % monos, total protein
45, glucose 50. Gram stain of the CSF shows many WBCs and no organisms seen. Is
this CSF analysis consistent with bacterial or viral meningitis? Which factors suggest
one or the other?
3. What are the three most common bacteria that cause meningitis and what
antibiotic covers them with close to 100% certainty?
4. Match the CSF results with the diagnosis (normal CSF, viral meningitis,
bacterial meningitis). Validate your answer. Assume that the patient is 6 months old.
CSF
White cells
%Neutrophils
CSF Glucose
Blood Glucose
Protein
CSF 1
1243
94%
23
78
62
CSF 2
5
1%
65
85
21
CSF 3
190
60%
50
87
48
CSF 4
250
86%
47
90
49
Answers to questions
1. This is most likely a viral meningitis. He is older, so his risk of bacterial
meningitis is lower. He has been fully immunized, which presumably means that he
has had H. influenzae, type B vaccine. He has probably had pneumococcal vaccine,
but this can't be automatically assumed. He is alert, ambulatory, and not toxic in
appearance, which all suggest that he does not have an overwhelming infection such
as bacterial meningitis.
2. This is most consistent with viral meningitis. Although he has a high
percentage of segs, this is still consistent with early viral meningitis. Cases of
bacterial meningitis which have not been pre-treated with antibiotics almost always
have more than 90% segs. The gram stain does not show any organisms which makes
bacterial meningitis less likely. This laboratory analysis of his CSF suggesting viral
meningitis, is consistent with his clinical appearance which also suggests viral
meningitis (see the answer to #1 above).
3. Pneumococcus, meningococcus and Haemophilus influenzae type B.
Pneumococcus is usually sensitive to penicillins and cephalosporins, but some
resistance has emerged so vancomycin should be given in addition to cefotaxime or
ceftriaxone. Meningococcus is sensitive to penicillin so cefotaxime or ceftriaxone
provides sufficient coverage. H. influenzae type B is sensitive to cefotaxime and
ceftriaxone, but this organism is not a common cause of bacterial meningitis due to
widespread immunization against this organism.
4. CSF 1 shows bacterial meningitis. The increased number of cells in the
CSF with a predominant number of neutrophils makes this a strong likelihood
possibility. In addition, he also has a very low glucose CSF level (CSF, blood
glucose ratio of 25%) and an increased protein value sometimes. Cases of early viral
meningitis can present with an increased number of cells and neutrophils but usually
the CSF glucose is normal or not lower than 40% of the blood CSF value.
CSF 2 is normal. The normal number of WBCs in the CSF depends upon the
age of the patient. The younger and more immature the infant is, the higher the value
is. CSF glucose value depends upon the value of glucose in the blood and upon the
integrity of the blood brain barrier. In patients with normal meninges the CSF value
is usually about 75% of the blood level. When the meninges become inflamed, the
active transport of glucose across the blood brain barrier becomes altered and the ratio
drops proportionately to the degree of inflammation. Most viral meningitis produce
less changes than bacterial meningitis accordingly CSF glucose values are lower in
bacterial meningitis.
CSF 3 shows viral meningitis. Most cases of viral meningitis will present
with a moderate increase in the number of white cells and a percentage of neutrophils
not higher than 60-70%.
CSF 4 is inconclusive. The high percentage of neutrophils indicates that
bacterial meningitis is possible. It would be wise to administer antibiotics until more
information can be obtained. The gram stain result will be helpful. If it is positive for
organisms, then this indicates bacterial meningitis. If the gram stain is negative,
bacterial meningitis still cannot be totally ruled out. The child's clinical condition is
not part of this table, but in reality, a child who is alert, active and playful is more
likely to have viral meningitis, as opposed to a lethargic, toxic child who is more
likely to have bacterial meningitis. This will probably turn out to be a case of viral
meningitis despite the high percentage of neutrophils, since an early viral meningitis
will often have high neutrophil percentages. A repeat LP 12 to 24 hours from the first
LP will be helpful. A repeat LP which demonstrates a clear shift toward mononuclear
cells, is consistent with viral meningitis, while no shift, or only a slight shift would
suggest bacterial meningitis. Culture of the CSF will be most definitive if it is
positive, but this result will not be available for at least 24 hours.
References
1. Dodge PR. Neurological Sequela of acute bacterial meningitis. Pediatr
Ann 1994;23:101-106.
2. Wubbel L, McCracken CH. Management of bacterial meningitis. Pediatr
in Rev 1998;19:78-84.
3. Schuchat A, Robinson K, Wengor JD, et al. Bacterial meningitis in the
United States, 1995. N Engl J Med 1997;337:970-976.
4. Feigin RD, Shackelford PG. Value of repeat lumbar puncture in the
diagnosis of meningitis. N Engl J Med 1973;289:571-574.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
UTI:
This is a 4 month old female who presents to the office with a chief complaint of
fever, vomiting, and loose stools. She has had tactile fever for 3 days, and had 5-6
episodes of emesis on the first day of illness. Stools were liquid on the first and
second days of illness. She was seen at an emergency room 2 days ago, where the
impression was gastroenteritis. No labs or x-rays were done in the emergency
department. She returns to the office now because of persistent fever. Vomiting and
diarrhea have resolved, but she is breast-feeding less well than usual. Her mother
notes that her urine seems "strong" and that she is not as playful as usual. She has had
no known ill contacts. She has no cough, URI symptoms, or rash. Past history is
unremarkable and she is on no medications.
Exam: VS T 38.9, P164, R40, Wt. 5.3kg (15%ile, and 150gm below her pre-illness
weight). She is alert, smiling, active, not toxic, and in no distress. Her anterior
fontanelle is soft and flat. Her eyes and ENT exams are normal. Her oral mucosa is
moist. Her neck is supple. Heart rate is regular without murmurs. Lungs are clear
and her respirations are non-labored. Her abdomen is flat, soft, non-tender, without
hepatosplenomegaly or masses. Her external genitalia are normal. Her skin is warm
and well perfused, with no rash. Her back exam reveals no deformities or cutaneous
defects. Her neurologic exam shows normal tone, strength, and activity.
A urine specimen obtained by transurethral catheterization yields a small amount of
cloudy urine, which is positive for leukocyte esterase and nitrite tests. This is sent for
culture. Her CBC shows a WBC 9.4, H/H 9.3/27.5, platelets 389,000, 51%
neutrophils, 44% lymphocytes, 3% monocytes, 2% eosinophils. She is given 250mg
of ceftriaxone intramuscularly and is scheduled for recheck in the office the next
morning.
At follow-up the next day, she is smiling and non-irritable, and shows a 250 gm
weight gain. She fed well overnight and continued to have a low grade fever. Urine
culture is positive for greater than 100,000 colonies/ml of a non-lactose fermenting
organism, with identification and sensitivities pending. Ceftriaxone is repeated at the
same dose. The following day, she is afebrile and her parents feel that she is entirely
back to normal. Urine culture result identifies E. coli, sensitive to all antibiotics
tested. She is started on oral trimethoprim-sulfamethoxazole (TMP-SMZ) to
complete 10 days of antibiotic therapy. She remains well on oral antibiotics.
Following 10 days of therapy, she is changed to a prophylactic dose of TMP-SMZ
and a renal ultrasound and voiding cystourethrogram (VCUG) are scheduled. Both
studies are normal. Repeat urine cultures on day 3 of antibiotics, and again at the time
of VCUG are negative. Antibiotics are discontinued. She has done well without
recurrent episodes of UTI.
Urinary tract infections (UTIs) are a common, potentially serious, and (especially in
young children) often occult bacterial infection of childhood. During childhood, UTI
occurs in approximately 3-5% of girls and 1% of boys. Most of the UTIs in boys
occur in the first year of life, whereas the age of the first diagnosed UTI in girls is
highly variable. After 2 years of age, UTI in females exceeds that in males by a factor
of 10:1 (1). Uncircumcised males less than one year old are more likely to be affected
than circumcised males (2,3). The prevalence of UTI in a febrile child 2-24 months
of age, without other source of infection, is 5% (4). After 6 years of age, and before
the onset of sexual activity, incidence of UTI falls dramatically in both sexes.
Many factors may predispose a child to UTI, including abnormalities of the urinary
tract such as vesicoureteral reflux (VUR), renal anomalies with hydronephrosis or
obstruction, neurogenic bladder, or nephrolithiasis; functional abnormalities such as
constipation, fecal incontinence, or incomplete bladder emptying; and environmental
factors such as bubble baths, poor perineal hygiene, pinworms, or sexual activity,
including sexual abuse. Labial adhesions in girls and phimosis in boys also contribute
to an increased risk of UTI.
UTI causes acute morbidity as well as long term sequelae including hypertension and
impaired renal function. Accurate diagnosis of UTI is important both to facilitate
appropriate management of the acute illness, and to insure appropriate evaluation and
follow-up. Equally important is accurately ruling out a UTI to avoid unnecessary,
costly, and potentially harmful treatment and evaluation.
The clinical presentation of UTI varies greatly, primarily with the age of the child. In
general, the older the child, the more clearly signs and symptoms point to the urinary
tract. Thus older children (over 6 years) and adolescents are likely to present with
dysuria, urgency, or frequency, and may have associated fever, chills, flank pain,
enuresis, or hematuria. Younger children (2-6 years) can have any of these same
signs and symptoms, but they may show more nonspecific signs such as abdominal
pain, altered voiding pattern, decreased appetite, or general malaise (5).
From infancy to 2 years of age, fever alone is the most common presentation of UTI
(6). There may be associated vomiting, diarrhea, constipation, poor feeding,
irritability, or late-onset jaundice, but these features do not aid in distinguishing UTI
from other causes of fever. Vomiting and diarrhea are frequently attributed to
gastroenteritis, when in fact it is a UTI. A history of malodorous urine or crying with
urination is helpful when present, but absence of these complaints does not rule out
UTI. In this age group, UTI should also be considered in the differential diagnosis of
failure to thrive. The possibility of UTI should be considered in any febrile (temp
greater than 39 degrees C, 102.2 degrees F) child under 24 months of age, keeping in
mind that girls under 24 months of age, and boys under 6 months of age are at highest
risk.
The diagnosis of UTI depends upon: first, maintaining a high index of suspicion for
the condition, especially in young children and infants; and second, performing
appropriate diagnostic studies.
Physical examination of the child with suspected UTI focuses first on assessing the
overall degree of illness severity (relatively stable or possibly toxic and septic) of the
child, including hydration status, level of alertness and comfort or discomfort, and
perfusion state. Vital signs must be evaluated, especially for fever, hypertension (as a
sign of renal impairment), signs of shock, and weight (for chronic failure to thrive or
acute weight loss suggestive of dehydration). The abdomen should be carefully
examined for any masses or tenderness, including costovertebral angle (CVA)
tenderness. Genitalia should be examined for signs of trauma, urethral or vaginal
discharge, labial adhesion, or phimosis. Rectal examination may provide further
assessment of any intra-abdominal masses or tenderness, and assessment of rectal
tone may aid in ruling out a neurologic abnormality which could contribute to UTI
susceptibility. Visual inspection of the sacral spine for skin dimples or other
cutaneous abnormalities may similarly lead the clinician to further evaluate the child
for spinal cord abnormalities associated with a neurogenic bladder.
The diagnosis of UTI requires culture of a properly collected urine specimen (7). In
children less than 2 years of age, a properly collected urine specimen requires an
invasive procedure: either suprapubic aspiration or transurethral catheterization. As
children advance in age and toileting abilities, it becomes possible to obtain a clean
catch mid-stream voided urine specimen and thus avoid invasive collection
techniques. A clean catch mid-stream urine sample means that the urethral meatus
and surrounding area should be clean, and that the urine collected should be from the
middle of the stream: i.e., the first few drops of urine should not be collected. For
girls, cleaning involves separating the labia and cleaning the area (usually with a
series of 3 pre-moistened antiseptic towelettes). For circumcised boys, the glans of
the penis should be similarly cleansed. For uncircumcised boys the foreskin is gently
retracted prior to cleaning. After cleaning, the child voids over the toilet, with the
parent "catching" the urine in a clean specimen cup after the first few drops are
passed. In girls this is often more easily accomplished by having the child sit facing
backwards on the toilet, so the parent can easily catch the urine stream from behind
the child.
Urinalysis (UA) is helpful in evaluating the likelihood of UTI, but cannot definitively
rule it in or out. The most readily available and useful components of the UA in this
context are the leukocyte esterase test, nitrite test, and microscopy. Sensitivity is
markedly improved when all three are used, although specificity is lower. A positive
leukocyte esterase or positive nitrite test is suggestive of UTI, as are more than 5
WBC per HPF (high power field) of a spun urine specimen, or bacteria present on a
gram stain of an unspun urine (a test not done by most labs unless specifically
requested).
Urine culture results are expressed quantitatively, indicating the colony-forming units
(CFU or colony count) of bacterial growth. The significance of a positive culture
depends upon the method of specimen collection and the number of colonies of a
single organism (8). In general, a colony count of greater than or equal to 100,000 is
considered positive on any properly obtained urine specimen. Colony counts of
greater than or equal to 10,000 on a catheterized specimen are also considered
positive. Colony counts of 1,000 to 10,000 on a catheterized specimen are suspicious
and should be repeated. A specimen obtained by suprapubic aspiration should be
sterile, so any growth of gram negative bacilli or any more than a few thousand gram
positive cocci is considered a positive culture.
Urine specimens obtained from young children by means of a bag applied to the
perineum have a high rate of contamination. A negative culture of a bag-collected
urine does rule out a UTI; however, a positive culture obtained in this way is not a
definitive diagnostic test. In fact, positive culture results from such a specimen are
estimated to be falsely positives as much as 85% of the time (7).
The most common causative agents of UTI are gram negative colonic bacteria, with
Escherichia coli being the cause of most acute UTIs (1,8). Klebsiella, Proteus, and
Enterobacter species are other common gram negative causes of UTI. Gram positive
organisms include Staphylococcus species and Enterococcus species. Cystitis may be
viral, usually caused by adenovirus (1,9).
UTIs are divided into two major classifications: those that involve the lower urinary
tract (cystitis) and those that involve the upper urinary tract (pyelonephritis). Lower
tract disease typically does not cause fever, and does not result in renal damage.
Upper tract disease classically causes fever, abdominal or flank pain, and in younger
children and infants the nonspecific signs of irritability, poor feeding, malaise, failure
to thrive, or vomiting and diarrhea. The differentiation of upper tract disease from
lower tract is primarily a clinical one, with supporting evidence provided by
technetium (Tc 99m) dimercaptosuccinic acid (DMSA) scanning (10) and by elevated
C-reactive protein (CRP) values (9).
The differential diagnosis of UTI varies with the age and presenting complaints of the
patient. The nonspecific signs associated with UTI in infancy and toddlerhood may
be associated with bacterial sepsis originating in any site, as well as with
gastroenteritis, hepatitis, or viral infection. Signs of cystitis in older children or
adolescents raise the possibility of chlamydial or gonorrheal urethritis. The
presenting complaints of pyelonephritis must be differentiated from acute
appendicitis, hepatitis, gall bladder disease, pelvic inflammatory disease, and other
causes of acute abdominal pain.
Treatment of UTI depends upon assessment of the likelihood of the diagnosis of UTI
and the clinical severity of the illness. These assessments will guide the clinician to:
await culture results before initiating antibiotic therapy; initiate empiric oral antibiotic
therapy; initiate empiric parenteral outpatient therapy; or hospitalize for empiric
parenteral therapy. Initial treatment decisions are made before culture results are
available, and are therefore empiric. The goals of prompt treatment are eradication of
the acute infection, symptom resolution, prevention of progression of disease (e.g., to
pyelonephritis, abscess, or sepsis), and reduction of the risk of renal scarring and its
long term sequelae (7,11).
When therapy is initiated empirically, the clinical condition of the child is the primary
factor considered. In every case, an adequate urine specimen for culture must be
obtained prior to initiating therapy. The younger and/or more clinically ill the child
with probable UTI is, the more aggressive initial therapy needs to be. In the non-toxic
appearing, usually older child, in whom there is a relatively low suspicion of UTI, and
no concern of upper tract disease, treatment may be deferred until urine culture results
are available. A non-toxic child, who is feeding well, is well-hydrated, and for whom
compliance and follow-up are not problematic, is appropriately managed with oral
antibiotics and close outpatient follow-up.
At any age, a child with signs of urosepsis, severe clinical illness, or significant
dehydration should be hospitalized for parenteral antibiotic therapy and close clinical
monitoring and supportive care. High risk children, such as those with immunologic
impairment or known urologic abnormalities, may also need hospitalization. Inpatient
therapy traditionally has been recommended for all children with suspected
pyelonephritis as well as for infants less than 1 year of age with UTI. Some of these
children may be managed with outpatient parenteral antibiotics, or even with oral
antibiotics (7,11,12), if compliance and close daily follow-up can be assured.
Children who are vomiting, or otherwise unable to reliably take oral medications, or
for whom compliance is a concern, should be treated parenterally (either as inpatients
or outpatients) until these issues are resolved (7,13).
The initial choice of antimicrobials is guided by the chosen route of administration,
known uropathogens, and any compromise of renal function of the patient. It is
adjusted based on clinical response and results of culture and sensitivity testing.
Initial oral therapy may be with a sulfonamide (TMP-SMZ or sulfisoxazole) or with a
cephalosporin (cephalexin or cefixime are commonly used). Parenteral therapy may
be with a cephalosporin (ceftriaxone, cefotaxime) or ampicillin and/or an
aminoglycoside (used with caution in the setting of impaired renal function). The oral
drug nitrofurantoin is excreted in the urine, but it does not reach therapeutic
concentrations in blood or tissues. It therefore should not be used to treat febrile UTIs
in infants, or to treat pyelonephritis.
The choice of initial oral empiric therapy involves consideration of spectrum, side
effects, allergies, palatability, dosage schedule, and price. TMP-SMZ is often
considered the drug of choice. It is, however, associated with some risk of StevensJohnson syndrome, and can precipitate hemolysis in patients with undiagnosed G6PD
deficiency. Cephalexin is the most palatable of the three, and the least expensive, but
usually dosed QID. Cefixime is the most expensive, but offers the advantage of once
a day dosage. TMP-SMZ is intermediate in price, and dosed BID. All three have
excellent coverage for the usual pathogens. Any of these drugs is an acceptable first
choice. Amoxicillin should no longer be considered a first line drug for empiric
therapy, due to increasing resistance of E. coli to amoxicillin/ampicillin.
Clinical response to therapy is generally prompt, with improvement evident within
24-48 hours of initiating antimicrobial therapy. If clinical improvement is seen, and
culture results indicate that the uropathogen involved is sensitive to the antimicrobial
being used, routine repeat culturing of the urine after two days of therapy is not
necessary. However, if sensitivities are unavailable, are intermediate or resistant, or
the expected clinical improvement is lacking, repeat culture should be obtained.
Children started on parenteral antibiotics may be changed to an oral antibiotic when
they are clinically well enough to do so. That is, when they are non-toxic, wellhydrated, afebrile, and tolerating oral intake. Again, oral antibiotic choice is guided
by the results of initial culture and sensitivity testing of the urine.
Duration of therapy varies somewhat, again based on age and degree of illness of the
child. Any child or infant with a febrile UTI needs a total of 7-14 days of antibiotic
therapy, with 10-14 days preferred for those with clinical evidence of pyelonephritis
(7). Short course therapy (3 days or less) is reserved for adolescent females with
uncomplicated cystitis (11).
The management of UTI does not end with the successful treatment of the acute
infection. Rather, it continues with the evaluation for renal anomalies or VUR,
monitoring for recurrence of UTI, short or long term antibiotic prophylaxis to prevent
recurrence, and medical or surgical management of any underlying predisposing
conditions.
Children with VUR are at increased risk of renal damage from UTIs, as are children
with other anomalies of the urinary tract. Therefore, all children (with the exception
of adolescent females with uncomplicated cystitis) with a documented UTI should be
investigated with a renal ultrasound and VCUG (14,15). These studies may be
performed as soon after the diagnosis of UTI as is convenient. Delaying studies for 36 weeks after the acute infection (as previously recommended) does not alter the
detection of VUR, but does substantially decrease the likelihood that the studies will
be completed (16).
If studies are delayed until after completion of 7-14 days of antimicrobial therapy, the
child should remain on antimicrobial prophylaxis until the studies are completed.
Drugs of choice include TMP-SMZ, sulfisoxazole, and nitrofurantoin, in doses
adjusted for prophylaxis rather than therapy (7,17).
The child with VUR needs long term follow-up with antibiotic prophylaxis, periodic
monitoring of urine cultures, repeat imaging of the urinary tract, and possible surgical
consultation (for persistent VUR, high grade VUR, or recurrent UTIs despite
prophylaxis) (14,18). DMSA scanning is helpful in determining the presence of renal
scarring in children with VUR and thus can assist in management decisions.
Prognosis after UTI in childhood depends on: 1) whether the infection was limited to
the lower tract (cystitis) or involved the upper tract (pyelonephritis), 2) the presence
or absence of VUR, and 3) the presence or absence of other urinary tract anomalies,
especially those with obstructive uropathy.
Uncomplicated infections without associated VUR or obstruction respond well to
antimicrobial therapy. However, as many as one third of these patients may
experience recurrence of UTI within the first year after acute infection (19). Followup urine cultures (generally monthly for 3 months, then at 3 month intervals X 3, and
then at 6 month intervals X 2) are therefore recommended.
VUR is present in 30-50% of children with UTI (20). Its severity is graded on a scale
of I, II, III, IV, V (14). While pyelonephritis and renal scarring can occur in the
absence of VUR, the severity of renal scarring correlates with the degree of reflux
(20). The natural history of low grade reflux is toward spontaneous resolution,
whereas high grade reflux is less likely to resolve without surgical intervention. The
combination of renal parenchymal infection (especially repeated infections) and VUR
or obstructive nephropathy puts children at risk for renal scarring which may progress
to chronic renal insufficiency, hypertension, reflux nephropathy, and end stage renal
disease (9,14,20). Early diagnosis and treatment of UTI and VUR or obstruction may
diminish the incidence of these long term complications (21).
In summary, appropriate management of UTI hinges on three essential factors, all of
which are the responsibility of the clinician: 1) Maintaining a high index of suspicion
for the diagnosis, especially in infants and toddlers who rarely have specific
symptoms, 2) Properly obtaining an adequate urine specimen for culture before
initiating antimicrobial therapy, 3) Following through on the patient's clinical
response, culture and sensitivity results, and the results of imaging studies and followup cultures. Careful attention to all of these points will optimize the diagnosis,
treatment, management, and outcome of the child with UTI.
Questions
1. When is it appropriate to treat empirically for UTI without first properly
obtaining an adequate urine specimen for culture?
2. What factors affect the decision of how to obtain a urine specimen when
UTI is being considered? How will the method of collection affect the interpretation
of culture results? When is a urine specimen obtained by bag collection a definitive
test for UTI?
3. What are some host and pathogen factors contributing to the development
of UTI?
4. How is pyelonephritis distinguished from lower tract UTI? What is the
importance of making the distinction?
5. What is the commonest clinical presentation of UTI in the child under 2
years of age? What are some associated signs and symptoms which may be present?
6. Which clinical features of UTI are reason to consider parenteral therapy
and/or hospitalization?
7. How would you explain to parent and child the technique of obtaining a
clean catch mid-stream urine sample: in girls and in circumcised and uncircumcised
boys?
8.
Familiarize yourself with the technique of transurethral bladder
catheterization (22) in male and female infants and toddlers, including: a) Prevention
of specimen contamination, b) Selection of appropriate equipment, c) Relevant
anatomic landmarks, and d) Possible complications.
Answers to questions
1. Empiric treatment for UTI should not be initiated without first obtaining an
adequate specimen for culture. The only pediatric exception would be a child so
severely ill (in septic shock and/or anuric) that waiting to obtain a urine sample could
be life threatening. One might consider empiric treatment without culture in an
uncomplicated older teen, however, such patients are rarely "uncomplicated" when
considering issues such as recurrence, sexually transmitted diseases, etc.
2. The method of obtaining a urine specimen is affected by the patient's age,
severity of illness, state of cooperation, toileting abilities, and whether or not
antibiotics are to be started empirically. The colony count considered positive varies
with the collection method: any growth with suprapubic aspiration; greater than or
equal to 10,000 CFU for a catheterized specimen; and greater than or equal to 100,000
CFU for a clean catch specimen. Bag specimens are only definitive when culture
result is negative (and therefore should not be used if empiric therapy is to be
initiated).
3. Host factors contributing to development of UTI include uncircumcised
male, labial adhesions, poor hygiene, constipation, urinary tract obstruction,
dysfunctional voiding patterns, and neurogenic bladder. Pathogens are those
commonly found in the vicinity of the urethra: skin and GI organisms, as well as
blood-born organisms in the neonate. The strains of E. coli which commonly cause
UTI show increased adherence to uroepithelial cells.
4. Classical signs of pyelonephritis include CVA tenderness, fever, and signs
of systemic illness, while lower tract disease is milder and may present with only
urinary urgency, frequency, or dysuria. Abnormal DMSA scan or elevated CRP
results support the diagnosis of pyelonephritis.
5. The commonest presentation of UTI in the child under two years of age is
fever. Associated signs and symptoms may include vomiting, diarrhea, irritability,
poor feeding, malodorous urine, oliguria, constipation, or jaundice.
6. Empiric parenteral therapy and/or hospitalization should be considered
when suspected UTI is associated with signs of urosepsis, severe clinical illness,
dehydration, immunologic compromise, or urologic abnormality. Vomiting, poor oral
intake, or concerns for poor compliance are also reasons to use parenteral therapy.
7. "Clean catch mid-stream" urine sample means that the urethral meatus and
surrounding area should be clean, and that the urine collected should be from the
middle of the stream: i.e., the first few drops of urine should not be collected. For
girls, cleaning involves separating the labia and cleaning the area (usually with a
series of 3 pre-moistened antiseptic towelettes). For circumcised boys, the glans of
the penis should be similarly cleansed. For uncircumcised boys the foreskin is gently
retracted prior to cleaning. After cleaning, the child voids over the toilet, with the
parent "catching" the urine in a clean specimen cup after the first few drops are
passed. In girls this is often more easily accomplished by having the child sit facing
backwards on the toilet, so the parent can easily catch the urine stream from behind
the child.
8a. Transurethral catheterization is an invasive procedure and is performed
using standard sterile technique, including povidone/iodine wash of the periurethral
and perineal areas, sterile field, sterile gloves, and sterile catheter and specimen cup.
8b. Infant feeding tubes in #5 or #8 french size are adequate for most infants
and toddlers. It is not necessary or advisable to use a Foley catheter, as there is no
need for a balloon. The catheter is removed as soon as the sample is obtained.
8c. The catheter is introduced into the urethral meatus, and advanced gently
until there is return of urine. This is done with the infant in the supine, "frog-leg"
position. The catheter tip may be lubricated with sterile lubricant or sterile water. In
circumcised boys the urethral meatus is easily seen. In uncircumcised boys it is
usually revealed by gentle retraction of the foreskin (if not, the foreskin is retracted as
far as is easily possible and the catheter introduced with gentle probing until the
meatus is located). The urethral meatus may be less easy to see in infant girls. It is
helpful to remember that it lies anterior to the vaginal introitus, and to be familiar with
the often fleshy appearance of the infant hymen. Separation of the labia, adequate
light, and familiarity with the appearance of the genitalia facilitate locating the
urethral meatus. A frequent error is introduction of the catheter into the vagina
(recognized by the absence of urine return and by resistance to gentle advancement of
the catheter beyond a couple of centimeters). Some practitioners opt in this situation
to leave the misdirected catheter in place while a second catheter is introduced into
the urethra (using the first catheter to "block" or mark the vaginal introitus). Whether
or not the first catheter is left in place, a new sterile catheter must be used for the
second attempt, to avoid contamination with vaginal flora.
8d. Complications of urethral catheterization include doubling back of the
catheter (either in the urethra or in the vagina), trauma to the urethral meatus or
mucosa, and possible introduction of infection. There can be subsequent stricture
formation. Familiarity with the anatomy and avoidance of any forceful catheter
advancement can minimize the risk of complications. A lubricated catheter of
appropriate size should advance easily through the urethra. Any resistance should be
taken as a sign to retract the catheter rather than to advance it more forcefully. The
risk of introduction of infection is minimized by careful adherence to sterile
technique.
References
1. Elder JS. Chapter 546 - Urinary Tract Infections. In: Behrman RE,
Kliegman RM, Jenson HB (eds). Nelson Textbook of Pediatrics, 16th edition. 2000,
Philadelphia: WB Saunders Company, pp. 1621-1625.
2. Wiswell TE, Roscelli JD. Corroborative evidence for the decreased
incidence of urinary tract infections in circumcised male infants. Pediatrics
1986;78:96-99.
3. To T, Agha M, Dick PT, Feldman W. Cohort study on circumcision of
newborn boys and subsequent risk of urinary-tract infection. Lancet 1998;352:18131816.
4. Hoberman A, Chao HP, Keller DM, et al. Prevalence of urinary tract
infection in febrile infants. J Pediatr 1993;123:17-23.
5. Steele RW. The epidemiology and clinical presentation of urinary tract
infections in children 2 years of age through adolescence.
Pediatr Ann
1999;28(10):653-658.
6. Roberts KB, Akintemi OB. The epidemiology and clinical presentation of
urinary tract infections in children younger than 2 years of age. Pediatr Ann
1999;28(10):644-649.
7. American Academy of Pediatrics. Practice parameter: The diagnosis,
treatment, and evaluation of the initial urinary tract infection in febrile infants and
young children. Pediatrics 1999;103:843-852.
8. Erratum: Table 2. Pediatrics 2000;105:141.(Correction of the table
published in reference #7).
9. Kraskinski KM. Chapter 36 - Urinary Tract Infections. In: Katz SL,
Gershon AA, Hotez PJ (eds). Krugman's Infectious Diseases of Children, tenth
edition. 1998, St. Louis: Mosby-Yearbook, Inc., pp. 605-617.
10. Heldrich FJ, Barone MA, Spiegler E. UTI: diagnosis and evaluation in
symptomatic pediatric patients. Clin Pediatr 2000;39(8):461-472.
11. Shaw KN, Gorelick MH. Urinary tract infection in the pediatric patient.
Pediatr Clin North Am 1999;46(6):1111-1124.
12. Hoberman A, Wald ER, et al. Oral versus initial intravenous therapy for
urinary tract infections in young febrile children. Pediatrics 1999;104(1):79-86.
13.
Fisher MC.
Pyelonephritis at home-why not?
Pediatrics
1999;104(1):109-111.
14. Sheldon CA, Wacksman J. Vesicoureteral reflux. Pediatr Rev
1995;16(1):22-27.
15. Kass EJ, Kernen KM, Carey JM. Paediatric urinary tract infection and the
necessity of complete urological imaging. BJU Int 2000;86(1):94-96.
16. McDonald A, Scranton M, et al. Voiding cystourethrograms and urinary
tract infections: How long to wait? Pediatrics 2000;105(4):E50.
17. Pomeranz A, El-Khayam A, et al. A bioassay evaluation of the urinary
antibacterial efficacy of low dose prophylactic antibiotics in children with
vesicoureteral reflux. J Urol 2000;164 (3 Pt 2):1070-1073.
18. Jodal U, Hansson S, Hjalmas K. Medical or surgical management for
children with vesico-ureteral reflux? Acta Paediatr Suppl 1999;88(431):53-61.
19. Nuutinen M, Uhari M. Recurrence and follow-up after urinary tract
infection under the age of 1 year. Pediatr Nephrol 2001;16(1):69-72.
20. Schlager TA. The pathogenesis of urinary tract infections. Pediatr Ann
1999;28(10):639-642.
21. Smellie JM, Prescod NP, et al. Childhood reflux and urinary infection: A
follow-up of 10-41 years in 226 adults. Clin Nephrol 1998;12:727-736.
22. Carlson DW, Digiulio GA, et al. Illustrated Techniques of Pediatric
Emergency Procedures. In: Fleischer GR, Ludwig S, et al (eds). Textbook of
Pediatric Emergency Medicine, 4th edition. 2000, Philadelphia: Lippincott Williams
& Wilkins, pp. 1856-1857.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
Febrile seizure
An ambulance brings a 15 month old boy to the emergency department with a seizure
associated with fever. He has been in good health except for a high fever that
developed today to about 103-104 degrees. His mother gave him a small dose of
acetaminophen. About 20 minutes ago when the mother was checking up on her child,
she noticed shaking of the arms and legs and his eyes had a blank stare. This went on
for what seemed like 5 minutes. She called 911 and an ambulance was dispatched. He
has been ill with a high fever today and a slight cough and mild nasal congestion. Just
prior to the seizure, he was playing with some toys. There is no vomiting, diarrhea,
rash, or fussiness.
Past medical history is unremarkable.
Family history is significant for an uncle who has epilepsy.
Exam: VS T 39.8 degrees C (103.6 degrees F), P 165, RR 30, BP 90/60, O2 sat 100%
on RA. He is clingy, alert to his surroundings, and otherwise is in no distress. His
mother appears anxious and there appears to be good bonding between her and her
child. Skin is without bruising or neurocutaneous stigmata. Anterior fontanelle is
closed. Pupils are equal and reactive. EOMs are conjugate. The red reflex is present
bilaterally. There is no sunsetting of the eyes. TMs are normal. His mouth exam
shows moist mucosa without erythema. The Brudzinski and Kernig signs are difficult
to assess. Respirations are regular. Neurologically, he moves both arms and legs
equally. His tone appears normal. The rest of the examination is normal.
Febrile seizures are broadly defined as seizures occurring in the presence of fever, but
in the absence of central nervous system (CNS) infection, in children ages 6 months
to 5 years of age. It is the most common reason for convulsions in children less than
5 years of age, and they occur in 2 to 5% of all children, although it has been reported
to be more frequent in Asian countries. In Japan, the rate has been reported to be 7%
and in the Mariana Islands 14%. It is thought that the rates in these areas are higher
because some of the common infections of childhood may occur earlier in life when
children are most susceptible to febrile seizures. Also, since more families sleep in
the same room, this may make recognition better than in Western countries (1). The
age at which febrile seizures most frequently occur is in the second year of life, and
they occur slightly more commonly in boys than in girls. Febrile seizures can be
divided into two types: simple and complex. Simple febrile seizures are
characterized by the following: duration less than 15 minutes, and generalized.
Complex febrile seizures have the following features: duration greater than 15
minutes, multiple within 24 hours, and/or focal (2).
The risk of recurrence after the first febrile seizure is about 33%, and about 9% will
have three or more recurrences. The risks for recurrence are: occurrence of the first
febrile seizure at a young age; family history of febrile seizures; short duration of
fever before the seizure; relatively low fever at the time of the initial seizure; and
possibly a family history of an afebrile seizure. It has been observed that the time of
recurrence is usually within the first year of onset. Although complex febrile seizures
are not usually associated with recurrent febrile seizures, they may be a risk factor for
epilepsy later in life. Febrile seizures seem to run in families, but their mode of
inheritance is unknown. The risk for other siblings developing febrile seizures is
about 10-20%, but may be higher if the parents also have a history of febrile seizures
themselves (2).
Febrile seizures usually occur in the first 24 hours of the onset of fever. It has been
suggested that it is the rapid rise in the child's temperature, which causes a febrile
seizure rather than the actual height of the fever itself; however, there is no substantial
proof to support this suggestion. The seizures are usually generalized and tonicclonic, but other types may be present as well. Parents may describe stiffening,
jerking, apnea, cyanosis and incontinence, usually followed by drowsiness
(commonly called post-ictal for short). There may be variations to this such as staring
without stiffness, jerking movements without prior stiffening, and localized stiffness
or jerking. Simple, benign febrile seizures should be short, usually 1 to 2 minutes, but
some may be longer (up to 15 minutes). Because of the short duration, medical
attention usually occurs after the seizure has ended (2).
Although the diagnosis of febrile seizure is likely in a 6 month to 5 year old with
fever and a convulsion, one should consider other causes such as meningitis,
encephalitis, Shigella gastroenteritis, medications/toxins (such as diphenhydramine,
tricyclic antidepressants, amphetamines, and cocaine), hypoglycemia, electrolyte
abnormalities (that could be due to dehydration), shaken baby syndrome, accidental
head trauma, and epilepsy (2). Many of these other diseases can be ruled out by a
good history, physical examination, and clinical appearance after the seizure has
ended.
How should these patients be managed in terms of diagnostic work-up and treatment?
Given that febrile seizures are a relatively common phenomenon, should every child
with fever and seizures have a lumbar puncture done to rule out meningitis, or CT
scan and EEG to look for CNS abnormalities? The American Academy of Pediatrics
attempted to answer this in two practice parameters on the evaluation and treatment of
children with febrile seizures that were published in 1996 and 1999 (3,4). The
recommendations of these two practice parameters are listed below. It should be kept
in mind that these are guidelines only and that each case should be individualized
according to the particular child, and the situation. One should remember that these
guidelines are written for practitioners with a wide range of experience and training;
therefore, the points mentioned here are meant to be conservative. Also these
guidelines are written for children from 6 months to 5 years of age who had a simple
febrile seizure and are neurologically normal. The guidelines do not include children
with complex febrile seizures.
1. Lumbar puncture. An LP should be strongly considered in all infants less than 12
months of age because signs and symptoms of meningitis may be minimal or absent
in this age group. An LP should be considered in children between 12 months to 18
months of age, since signs of meningitis might be subtle. An LP does not need to be
done in children older than 18 months unless they show signs of meningitis (neck
stiffness, Brudzinski and Kernig signs) or have symptoms of a CNS infection. Infants
and children who were treated with antibiotics prior to the seizure should be strongly
considered to have an LP done. This is because antibiotics can mask the signs and
symptoms of meningitis (partially treated meningitis). Even if an LP is performed
and the results are negative, it is still prudent to be cautious and vigilant since spinal
fluid may be normal in the early stages of meningitis (3). The clinical appearance of
the child after the seizure has ended plays a very significant role, in that the playful,
active child who appears normal, probably does not have meningitis.
2. EEG. An EEG does not need to be performed as part of the work-up for a first
time simple febrile seizure. Although the EEG may be abnormal (occipital slowing)
in the first month after the seizure, there has been no correlation of this to recurrence
risk or the risk for developing epilepsy in the future (3,5). An EEG done 4 to 6 weeks
following the seizure should be normal. If it is not, then epilepsy is more likely. In
clinical practice this is not usually done after a single simple febrile seizure since an
EEG is difficult to do in young children and 4 to 6 weeks have passed, presumably
without any more seizures.
3. Laboratory studies. Laboratory tests, such as a CBC, serum electrolytes, calcium,
magnesium, phosphorus, and glucose, need not be done routinely. It should instead
be tailored to the presenting symptoms. For example, electrolytes and glucose can be
checked in a patient who is vomiting.
4. Neuroimaging. CT scan of the head does not need to be done routinely. There is
no data available showing that children with febrile seizures have an increased
incidence of CNS abnormalities, nor any evidence that febrile seizures lead to
structural brain damage.
Recommendations are not as clear-cut for children who develop complex febrile
seizures; however, the threshold for performing blood tests, neuroimaging, and EEG
is lower. A lumbar puncture should be strongly considered if the patient is young, if
there are signs and symptoms of meningitis, if the patient is already on antibiotics, if
there is no rapid improvement, or if the patient does not regain full consciousness (5).
If the child develops another seizure, then supportive measures are recommended.
During the time the seizure is occurring, the patient should be placed on his/her side
to prevent aspiration, and the airway should be maintained. Also nothing should be
placed in their mouths. If it is prolonged, then diazepam (Valium) should be given
either intravenously or rectally. If the patient has a fever, avoiding overheating by
removing blankets and heavy clothes can prevent febrile seizures, in addition to
administering antipyretics such as acetaminophen and giving cool baths. Diazepam
can also be used to prevent future recurrences of febrile seizures for the next several
hours, although its administration as a preventive measure is controversial (5).
Should a patient be hospitalized? Probably not, although it depends what the
circumstances are. It is recommended that patients who had a febrile seizure be
observed in the emergency department for several hours and reevaluated. After this
time, most children would have improved, and if the cause of the fever is known and
treated, they can then be sent home. Similarly, in a doctor's office, they can be
observed if they are rapidly improving. If they are not improving, then the diagnostic
studies mentioned previously should be considered. Circumstances when they should
be hospitalized for overnight observation are: the clinical situation is still unstable,
there is a possibility of meningitis, and/or the parents are unreliable or unable to cope
with the child developing another seizure (1).
An essential component of management is parental counseling. Reassurance should
consist of three components. First, parents should be reassured by informing them
that although the febrile seizure is frightening, it will not cause brain damage, and the
possibility of their child developing epilepsy is small. Secondly, they should also be
told that there is a possibility that it could happen again, especially in the first 24
hours. Also one third of children will have at least another febrile seizure later, with
most occurring within one year of the episode. Thirdly, if a seizure occurs, the child
should be kept on his/her side, and they should observe their child. If the seizure does
not stop in 3 minutes, then emergency medical services should be contacted (1).
Long-term pharmacotherapy is probably unnecessary, especially for simple febrile
seizures. Although the AAP Practice Parameters discourage use of anticonvulsants in
simple febrile seizures, it does mention that giving diazepam during the start of fever
in patients with parents having high anxiety is an option. Diazepam is given orally
using a dose of 1 mg/kg/day in three divided doses when the child is febrile.
Disadvantages of using diazepam are lethargy, drowsiness, ataxia, and masking of a
CNS infection. Other medications that have been used to prevent recurrences are
phenobarbital and valproic acid. A dose of 5 mg/kg/day of phenobarbital is given
once to twice a day. Although they can prevent 90% of recurrences of febrile
seizures, they are not without significant side effects. Phenobarbital has been
associated with behavioral problems (hyperactivity) and hypersensitivity reactions.
Valproic acid has a risk of developing fatal hepatotoxicity, thrombocytopenia, weight
changes, gastrointestinal problems, and pancreatitis. These medications have been
considered in those patients who have focal paralysis after a seizure, multiple seizures
in a young child, and high parental anxiety despite reassurance (1,4). Phenytoin and
carbamazepine have no demonstrated efficacy in preventing febrile seizures.
Despite the frightening appearance of the episode, and the parental belief that their
child is going to die, simple febrile seizures remain a benign condition with the
majority of children having no neurological sequelae. In other words, it does not lead
to brain damage or cognitive abnormalities. Although the risk of developing another
febrile seizure is moderate, the possibility of epilepsy is very small. For this reason,
long-term therapy anticonvulsant therapy is not usually recommended, but
practitioners should provide reassurance, education of what to do when their child has
another febrile seizure, and antipyretic therapy when a fever is present.
I would like to thank Dr. Yoshio Futatsugi for reviewing this chapter and Dr.
Robert Bart for his helpful suggestions and input.
Questions
1. At what ages do febrile seizures occur? How common is this problem?
2. In what percentage of patients will febrile seizures occur a second time?
3. What are the differences between simple and complex febrile seizures?
Why is it important to know this distinction (think of recurrence risk of febrile
seizures, development of epilepsy, and work-up)?
4. A febrile seizure is a diagnosis of exclusion. What other diagnoses should
be considered in a child with fever and seizures?
5. According to the guidelines put forth by the American Academy of
Pediatrics' Practice Parameter, who should be strongly considered to receive a lumbar
puncture?
6. Most patients with febrile seizures can be discharged home. What are three
indications for a child who should be hospitalized for overnight observation?
7. Although diazepam (Valium) can be used to prevent recurrences when
given at the start of a febrile illness, what are its disadvantages?
8. A key part to management is reassurance. What are three ways parents
should be reassured and educated?
Answers to questions
1. 6 months to 5 years. It occurs in 2-5% of all children and is the most
common reason for convulsions in children less than 5 years of age.
2. 33%.
3. Simple seizures are characterized by being less than 15 minutes duration
and generalized. Complex febrile seizures are greater than 15 minutes duration,
multiple within 24 hours, and focal. Simple febrile seizures have a higher risk for
febrile seizures. Complex febrile seizures have a higher risk for epilepsy. One should
have a lower threshold for performing tests and hospitalization in cases of complex
febrile seizures.
4. Meningitis, encephalitis, Shigella gastroenteritis, medications and toxins,
hypoglycemia, electrolyte abnormalities, shaken baby syndrome, accidental head
trauma, and epilepsy.
5. Infants less than 12 months of age.
6. Unstable clinical situation, possibility for meningitis, and parents unreliable
or unable to cope with the child developing another seizure.
7. Disadvantages include lethargy, drowsiness, ataxia, and masking of a CNS
infection.
8. 1) Seizure will not cause brain damage and the risk of a child developing
epilepsy is small. 2) Possibility that it can happen again, especially in the first 24
hours. One third of children will have at least another febrile seizure with most
occurring within one year of the episode. 3) If seizure occurs again, child should be
kept on his or her side. If seizure does not stop within 3 minutes, then emergency
medical services should be contacted.
References
1. Aicardi, J. Chapter 15 - Febrile Convulsions. In: Aicardi, J. Epilepsy in
Children, second edition. 1994, New York: Raven Press, pp. 253-275.
2. Hirtz, DG: Febrile Seizures. Pediatrics in Review 1997; 18(1): 5-9.
3. Provisional Committee on Quality Improvement, Subcommittee on Febrile
Seizures: Practice Parameter: The Neurodiagnostic Evaluation of the Child With a
First Simple Febrile Seizure. Pediatrics 1996; 97(5): 769-772.
4. Committee on Quality Improvement, Subcommittee on Febrile Seizures:
Practice Parameter: Long-term Treatment of the Child With Simple Febrile Seizures.
Pediatrics 1999; 103(6): 1307-1309.
5. Verity CM. Chapter 15 - Febrile Convulsions: A Pragmatic Approach. In:
Porter RJ and Chadwick D (eds). The Epilepsies 2, 1997, Boston: ButterworthHeinemann, pp. 289-311.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
Cerebral palsy
Roy is a 13 month old male who has been followed in the Pediatric outpatient clinic.
He was born at term by normal vaginal delivery without complications and his birth
weight was 3300g. His mother did not have any problems during the pregnancy. At 6
months of age, you noticed his head control was poor. Currently, he calls everyone
"mama" and follows one-step commands. He is able to drink from a cup. He is able
to roll over from his stomach to his back but he is not able to sit or stand. His height
and weight are both between the 25-50th percentiles and his head circumference is
within 2 standard deviations of the mean. Some primitive reflexes such as the
Asymmetric Tonic Neck Reflex (ATNR) persist and he has increased muscle tone,
especially in his legs. His deep tendon reflexes are exaggerated.
Cerebral palsy (CP) is defined as a non-progressive, but often clinically changing
motor impairment due to an abnormality of the developing brain. It is a symptom
complex or a descriptive term rather than a specific disease. Intellectual, sensory,
and/or behavioral problems may also exist although the primary abnormality must be
a motor deficit. The prevalence is estimated at about 2 per 1000 early school aged
children (1).
In more than 50% of the children who have CP, an etiology may not be evident (1).
The insult to the brain may occur prenatally (e.g., congenital malformation,
intrauterine infections, teratogens), perinatally (e.g., birth trauma, anoxia), or
postnatally (e.g., infections, accidental or non-accidental trauma, intracranial
hemorrhage). The majority of the cases are not caused by hypoxic ischemic incidents
occurring perinatally as it was believed until recently. 70-80% cases are prenatal in
origin (3). Infants who weigh less than 1500g at birth have a 10% to 20% risk of
developing CP (2). Although prematurity is the most common known antecedent of
CP, the majority of children who develop CP are born at term.
CP is often classified according to the predominant type of motor impairment:
spastic, dyskinetic, ataxic, or mixed. Spastic CP is the most common type and affects
70-80% of individuals with CP. It is characterized by a generalized increase in
muscle tone. CP can be further classified based on which limbs are involved, the
suspected etiology or functional capacities. For example, in spastic diplegia, the
lower extremities are more involved than the upper extremities. In hemiplegia, one
side of the body is more involved. All the extremities and often trunk and oral motor
function are also affected in spastic quadriplegia.
Choreoathetoid CP is a subtype of dyskinetic CP. Athetosis are slow writhing
involuntary movements and involves distal limbs. Choreiform movements are
asymmetric, uncoordinated, involuntary muscle contractions. These movements are
more prominent under stress and their intensity may change. It may not be apparent
until about 12 to 18 months of age when a toddler starts to show athetoid or dystonic
posturing on voluntary movements. One known cause of this form of CP is
encephalopathy associated with very high bilirubin levels during the neonatal period.
Ataxic CP is characterized by cerebellar dysfunction. This is the least common type
with a frequency of 1% among individuals with CP. Mixed CP involves both
symptoms of upper motor neuron and extrapyramidal symptoms. For example, a
child who has spastic quadriplegia may also have choreoathetoid movements.
The diagnosis of CP is essentially clinical and depends on knowledge of normal
development and its variation. While no factors or combination of factors is an
absolute predictor of CP, certain situations warrant closer monitoring. It is also
important to remember that the neurological picture may change as the child grows
older and the CNS matures. It is often difficult to diagnose children with CP before 6
months of age.
During infancy, feeding difficulty is an important sign. A child may continue to need
gavage (tube) feedings. He or she may be difficult to feed, or require an excessive
amount of time for feeding. A child may have failure to thrive or a poor rate of head
growth due to a serious insult to the brain. Constipation is another symptom among
infants with CP. He/she may be quiet and very easy, or irritable during infancy. The
child may show a premature handedness preference during the first 18 months of life.
This can be an early sign of hemiplegia.
There are several useful parameters for the assessment of neuromotor function.
A) Muscle function: Muscle tone and strength should be examined. By
simple observation, you may be able to see poor head control, scissoring of the lower
extremities, or flexor posturing of upper extremities.
B) Patterns of movement should be assessed. There are three patterns of
movements: 1) Normal movements, 2) Abnormal movements which are never seen in
normally developing children (e.g., restricted movements in children with spastic
diplegia or hemiplegia, or involuntary movements seen in children with athetoid CP),
3) Atypical movements which may be seen in normally developing children (e.g.,
bouncing along the floor while supine, or log-rolling as methods of mobility).
C) Structure or alignment of the body. A child with spastic CP may have
dislocation of the hip due to adductor and abductor muscle tone imbalance and
resultant poor joint development. Children with spastic CP also have the tendency for
plantar flexion of the feet. Scoliosis can be a problem for all children with CP.
D) Reflexes should be assessed. Physicians are generally very familiar with
the deep tendon reflexes. Of equal significance are so-called developmental reflexes.
Children with CP may have persistent primitive reflexes such as the Moro reflex and
the asymmetric tonic neck reflex (ATNR). Also, children with CP may present with
delayed emergence of righting reactions (natural tendency to position the body/head
upright) and protective/equilibrium responses (e.g., parachute reflex) as signs of
delayed maturation or CNS injury.
E) Gross motor skills are usually affected but other developmental milestones
should be also assessed to determine if delays are more global.
Many co-existing conditions are frequently seen in children with CP. These may
include sensory impairments, seizures, cognitive impairment, orthopedic problems,
impaired speech and language, feeding issues, dental problems, skin breakdown and
respiratory infections. Because CP is the result of an insult to the developing brain,
some of these problems may not be treatable or they may only partially respond to
medical/surgical treatment. The treatment plans and programs must be individualized
and modified over time as the child grows.
Children with CP have a high incidence of visual impairments (4,5). They may have
refractive errors, visual fields defects, or cortical blindness. Strabismus is very
common and may lead to the development of amblyopia. There is also an increased
incidence of sensorineural and conductive type hearing impairment. Hearing
impairments can further delay speech and language development of children with CP.
25-50% of children with CP may also experience seizures (4,5). Seizures are most
commonly seen among the children with spastic quadriplegia and hemiplegia.
Generalized tonic-clonic and partial seizures are the common types. Approximately
half of the children with CP have mental retardation. Although children with more
severe motor involvement tend to have mental retardation more frequently, this is not
always the case. Among children with normal intelligence, there is a higher incidence
of learning disabilities. Feeding difficulties (e.g., with sucking, chewing, and/or
swallowing) are common among children with CP as a result of impairment of oral
motor muscle function. This may also cause problems with speech articulation.
Drooling and gastroesophageal reflux may also occur. Aspiration can cause
pneumonia which is the leading cause of death in children with CP.
Because of the difficulties in motor control, assistance is needed to maintain good
posture and alignment and good range of motion of the joints. Subluxation or
dislocation of the hip are common in children with CP. The incidence is higher
among children with spastic quadriplegia. Dislocated hips can develop arthritis and
severe pain. Poor posture or positioning can result in scoliosis due to the unequal
muscle tension. Spasticity and limited muscle use may lead to contractures. The
interventions used to treat these conditions include physical therapy, orthopedic
surgery, muscle tone management (e.g., intrathecal Baclofen infusion), and orthoses
(e.g., ankle-foot orthosis, body shell).
The life expectancy of children with CP depends on the type and the severity of the
condition. Although the projected life span of children with CP is less than that of the
general population due to complications of motor dysfunction, the majority of
affected children will survive well into adulthood if given appropriate medical
attention.
The prognosis regarding ambulation is also dependent on the type and the severity of
the motor dysfunction. Overall, children with hemiparesis will walk by 18 months to
36 months. With or without assistive devices, 80-90% of children with diplegia, 70%
of children with dyskinesia and 50% of children with quadriplegia may achieve some
degree of ambulation (1). Ambulation may be predicted based on the achievement of
motor milestones. For example, there is a good prognosis for attaining some
ambulation if a child is able to sit independently by 24 months.
Because of multisystem involvement and various psychosocial and medical needs, no
one discipline can assess and manage all aspects of the child with CP alone. It is
important to be interdisciplinary to have a successful management program. The
child's physician has to be familiar with community resources such as early
intervention programs and support groups. It is important for the primary care
physician to communicate with the therapists, specialists, and school personnel. The
physician needs to advocate for the necessary services for the child and his/her family.
Primary care physicians should be aware of the different problems and needs that the
children experience as they get older and help them transition from the toddler to
school age to adulthood as smoothly as possible. The goal for the treatment program
is to maximize function and optimize development to help them participate in as
many activities as possible in multiple social settings.
Questions
1. Cerebral Palsy may have changing clinical features in: (select one)
a. The brain abnormality.
b. Effects on the motor system.
c. That seizures are usually not treatable after a few years.
d. Subtypes - first a person has the choreoathetoid type, then the spastic type,
and then becomes quadriplegic.
e. Decrease in IQ over time.
2. Currently, most cases of cerebral palsy with a known etiology are thought to be
a. Prenatal in origin.
b. Perinatal in origin.
c. Postnatal in origin.
3. What is the most common type of cerebral palsy?
a. Spastic
b. Choreoathetoid
c. Ataxic
d. Mixed
e. All are equally common
4. Which of the following is NOT a worrisome sign that may indicate cerebral palsy?
a. Poor rate of head growth
b. Hand preference at 6 months of age
c. Scissoring of the legs
d. Obesity
e. High muscle tone
5. True/False: Because of the neuromotor dysfunction and associated conditions,
children with cerebral palsy rarely live into adulthood.
6. True/False: Children with hemiplegia have a higher rate of ambulation than
diplegia and quadriplegia
Answers to the questions
1.b, 2.a, 3.a, 4.d, 5.false, 6.true
References
1. Taft LT. Cerebral palsy. Pediatr Rev 1995;16(11):411-418.
2.
Kuban KCK, Leviton A.
Cerebral Palsy.
New Engl J Med
1994;330(3):188-195.
3. Miller F, Bachrach SJ. Cerebral Palsy: A Complete Guide for Caregiving.
1998, Baltimore: John Hopkins University Press.
4. Blackman JA. Cerebral Palsy. In: Wolraich ML (ed). Disorders of
Development and Learning: A Practical Guide to Assessment and Management, 2nd
edition. 1996, St. Louis: Mosby, pp. 186-212.
5. Swaiman KF, Russman BS. Cerebral Palsy. In: Swaiman KF, Ashwal S
(eds). Pediatric Neurology: Principles & Practice, 3rd edition. 1999, St. Louis:
Mosby, pp. 312-324.
6. United Cerebral Palsy Associations, Inc. (www.ucpa.org)
7. American Academy for Cerebral Palsy and Developmental Medicine
(www.aacpdm.org)
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
Nephritis:
A 7 year old male presents to his primary care physician with the chief complaint of
dark "cola colored" urine, facial puffiness and abdominal pain for the past 2 days. He
had been in his usual state of good health until 14 days ago when he had a sore throat
and fever. His sore throat and fever resolved. He was not seen by a physician at that
time. Over the past 2 days facial puffiness has been noted, but no swelling of his
hands or feet. He has had some nonspecific abdominal pain that comes and goes
which does not seem to be related to eating or bowel movements. There is no nausea
or vomiting. His urine is dark brown and he has not been voiding as much as usual,
only 2 times in the past 24 hrs. There is no urinary frequency, urgency, dysuria or
foul smell to the urine. His appetite has been poor although he is still drinking fluids
well. He is also complaining of some back pain in the flank area that he describes as
a dull pain that comes and goes and does not seem to be related to activity. His
energy level is down and he has not felt up to going to school for the past 2 days. He
is also complaining of a dull generalized headache that has not been relieved with
acetaminophen.
Review of systems is negative for recent skin infection, skin rash, cough, rhinorrhea,
seizure activity, fever, arthralgia or weight loss. His past medical history, family
history and social history are unremarkable.
Exam: VS T 37, P 100, RR 20, BP 120/75, oxygen saturation 100% in RA. Height
and weight at 50th %tile. He is tired appearing but in no acute distress. Pupils are
equal and reactive. Optic disc margins are sharp. Sclera are white and conjunctiva
are clear. Mild periorbital is edema noted. TMs are normal. Throat, oral mucosa and
nose are normal. His neck is supple without lymphadenopathy. Heart is regular
without murmurs. Lungs are clear. Abdomen is diffusely tender (mild), without
guarding or rebound. Bowel sounds are normal. No organomegaly is noted. Mild
CVA tenderness is present. His extremities are warm, with strong pulses. Capillary
refill is less than 2 seconds. No edema is noted in his legs, feet or hands. No skin
rashes or impetigo scars are noted. His genitalia are normal. No scrotal edema is
present. Neurologic exam is normal.
Lab: His urine is tea colored. UA shows an increased specific gravity. A dipstick is
positive for a large amount of blood and moderate protein. RBCs are too numerous to
count. 5-10 WBCs per HPF. RBC casts are present. CBC with diff is normal.
Throat swab is sent for culture. ASO titer is elevated. Serum complement C3 level is
low. Serum electrolytes are normal. BUN 23 and Cr 0.8.
Clinical Course: He is diagnosed with acute poststreptococcal glomerulonephritis.
He is initially hospitalized for treatment of oliguria/volume overload with furosemide,
and monitoring of his modest hypertension. He has a good urine output with the
furosemide, however he later requires a calcium channel blocker to control worsening
hypertension. He is placed on a fluid and sodium restricted diet. His throat culture
later returns positive for group A beta hemolytic streptococci (GABHS), so he is
given a course of penicillin. He is discharged after 3 days of hospitalization. His
hypertension resolves over the next 2 weeks. He is followed closely by his primary
physician and his proteinuria and gross hematuria resolve early. His C3 level
normalizes two months after the onset of illness. Microscopic hematuria is expected
to persist for months so this will be rechecked in 3 to 6 months. He does not develop
any long term complications.
Acute glomerulonephritis (GN) presents with hematuria, oliguria, hypertension and
volume overload (edema), which are the findings of the classic "nephritic syndrome".
Acute GN (AGN) is associated with inflammation and proliferation of the glomerular
tuft. Most AGN is immunologically mediated. In acute poststreptococcal
glomerulonephritis (APSGN), immune complexes form with streptococcal antigens,
localize on the glomerular wall, activate the complement system, and initiate a
proliferative and inflammatory response. AGN may be rapidly progressive (RPGN).
Chronic GN (CGN) implies that permanent damage has occurred.
Acute poststreptococcal glomerulonephritis (APSGN) is the most common form of
glomerulonephritis in children. APSGN can occur in all ages but is most frequent in
males between 5 and 15 years. APSGN can occur after either an upper respiratory
tract or skin infection due to GABHS. It is more common after an infection of the
throat. CGN occurs more often in teenagers and adults. There are genetic
predispositions for familial GN (Alport, X-linked) and autoimmune etiologies (e.g.,
SLE-lupus nephritis).
Goodpasture's disease (anti-basement membrane
autoantibodies) also presents with a classic nephritic syndrome in conjunction with
hemoptysis, but this condition is rare.
Important questions to ask the patient/caregiver include history of macroscopic
(gross) hematuria (tea or cola colored urine, or red colored urine), sore throat,
impetigo, prior URI at least 1 week previously or skin sores (impetigo) in the
preceding 3-4 weeks (suggestive of APSGN), URI in the preceding few days
(suggestive of IgA nephropathy), reduced urine output, dyspnea, fatigue, lethargy,
headache or seizures (hypertensive encephalopathy). Also, symptoms of a systemic
disease such as fever, vasculitic rash (especially on the buttocks and legs posteriorly),
arthralgia and weight loss may be present. On physical exam, pay particular attention
to hypertension, pallor, signs of volume overload (edema, jugular venous distention,
hepatomegaly, crackles in the lung bases), impetigo and rash. For PSGN, edema
(specifically, facial edema involving the periorbital area) is the most frequent
presenting symptom. Dark colored or bloody urine is frequently not noticed by
patients because the abnormal color is only visible when the urine is collected in a
cup. The abnormal color is not noticeable in a urine stream unless the urine color is
very dark.
Many patients with APSGN are asymptomatic and do not seek medical care. Mild
hypertension is often asymptomatic. The classic dark urine is often not noticed.
Screening urinalysis may often identify persistent microhematuria which eventually
resolves months later. Many of these cases are felt to be resolving APSGN cases
which never presented for medical attention during the acute nephritis phase.
Throat culture for GABHS will be positive in 15-20% of patients with APSGN. CBC
is normal in AGN and with chronic renal insufficiency a normocytic normochromic or
hypochromic microcytic anemia will usually be found. Serum chemistries will reflect
the degree of renal failure (BUN, creatinine, potassium and phosphate are all elevated,
while calcium is decreased), which is usually mild. The ASO titer will be positive in
60% of patients with APSGN. The complement C3 serum level will be low in
APSGN and in other causes of GN described below. Urine microscopy shows RBC
casts and crenated RBCs in AGN. EKG, CXR and renal ultrasound are other tests
that should be considered. RBC casts indicate the presence of acute nephritis. WBC
casts can also be seen in APSGN, interstitial nephritis and pyelonephritis.
During convalescence from APSGN, complement C3 levels return to normal within
6-8 weeks. Persistently low C3 levels indicate an etiology other than APSGN. Gross
hematuria will generally resolve within 1 to 2 weeks. Microscopic hematuria may
persist for a year or more.
The differential diagnosis for glomerulonephritis includes infectious etiologies such
as GABHS, pneumococcus, mycoplasma, mumps and EBV. Glomerulonephritis may
also be related to hepatitis B and C as well as syphilis infections. IgA nephropathy,
membranoproliferative GN, autoimmune GN, familial GN, acute interstitial nephritis,
hemolytic uremic syndrome and pyelonephritis should all be on your differential
diagnosis list. One way to sort out the etiology of the glomerulonephritis is to look at
the complement level and whether evidence of systemic or renal disease is present.
For a patient with low serum complement level and systemic disease consider
vasculitis and autoimmune disease (SLE), subacute bacterial endocarditis, shunt
nephritis and cryoglobulinemia. For a patient with low serum complement level and
evidence of renal disease consider APSGN and membranoproliferative
glomerulonephritis (types 1,2, and 3). In a patient with normal serum complement
level and evidence of systemic disease consider polyarteritis nodosum, Wegener
vasculitis, Henoch-Schonlein purpura and hypersensitivity vasculitis. In a patient
with normal serum complement and evidence of renal disease consider IgA
nephropathy, idiopathic RPGN and immune complex disease. A renal tumor (e.g.,
Wilms) will occasionally present with gross hematuria so an imaging study may be
indicated to rule this out.
APSGN is a self-limiting disease. Treatment is symptomatic. Restricted fluid and
sodium diets are initially beneficial. Potassium and phosphate may also need to be
restricted. Medication may be required for management. Loop diuretics (e.g.,
furosemide) are the first choice for volume overload, hypertension and hyperkalemia
control. Vasodilators such as calcium channel blockers are also used to manage
hypertension. IV antihypertensives may also be required to treat severe refractory
hypertension. In severe hyperkalemia serum potassium lowering agents and IV
calcium may be needed. Immunosuppressive agents are used in the treatment of
vasculitis associated GN, membranoproliferative GN and RPGN. Plasmapheresis
may be used to treat RPGN.
Most patients with APSGN do not need hospitalization.
Indications for
hospitalization include: an uncertain diagnosis, significant hypertension, anticipated
poor follow-up, cardiovascular or cerebrovascular compromise, etc. The diagnosis
can usually be firmly established as an outpatient. An imaging study may be
necessary to rule out a Wilms tumor. If the patient's blood pressure is normal or only
mildly elevated, most parents can be taught to measure the child's blood pressure at
home using an automated blood pressure measurement device which is easily
available at most stores. Parents must notify the physician when the blood pressure
exceeds the parameters given by the physician. If the parents are deemed to be
unreliable, or are not capable of measuring the child's blood pressure, then
hospitalization should be considered.
Prognosis is excellent for APSGN and variable for other causes of GN in children.
Complications of AGN include acute renal failure, hyperkalemia, hypertension,
volume overload (congestive heart failure, pulmonary edema, hypertension) and
chronic renal failure.
Questions
1. When does the complement C3 level return to normal in APSGN?
2. What is the significance of finding red cell casts in the urine?
3. What is the significance of finding white cell casts in the urine?
4. How long does hematuria persist in APSGN?
5. Describe some indications for hospitalization of patients with APSGN.
6. What are the clinical elements of the nephritic syndrome?
7. What are classic causes of the nephritic syndrome?
8. A 5 year old boy has a screening urinalysis as part of a general physical
exam. The UA shows microscopic hematuria. History suggests that he has impetigo
periodically. What a likely cause for the microscopic hematuria?
Answers to questions
1. C3 levels return to normal within a 6-8 week period in APSGN.
Persistently low C3 levels suggest a cause other than APSGN.
2. The presence of red cell casts on urinalysis almost always indicates the
presence of glomerulonephritis. They can also be seen after strenuous exercise and
renal trauma.
3. The presence of white cell casts on urinalysis can be seen in APSGN,
interstitial nephritis and pyelonephritis.
4. Gross hematuria resolves within days to weeks. Microhematuria may
persist for months.
5. An uncertain diagnosis, significant hypertension, anticipated poor followup, cardiovascular or cerebrovascular compromise, etc.
6. Gross hematuria, oliguria, hypertension, edema (usually mild).
7. APSGN and Goodpasture’s. Other causes of nephritis include SLE
nephritis, MPGN, RPGN, Alport's, etc.
8. Convalescing APSGN.
References
1. Ruley EJ. Chapter 230 - Nephritis. In : Hoekelman RA(ed). Primary
Pediatric Care, 2nd edition. 1992, St. Louis: Mosby-Year Book, Inc., pp. 1379-1386.
2. Bergstein JM. Chapter 18 - The Urinary System, Nephrologic Diseases.
In: Behrman RE, et al (eds). Nelson Textbook of Pediatrics, 14th edition. 1992,
Philadelphia: W.B. Saunders Company, pp. 1323-1336.
3. Travis LB. Chapter 19.6 - Glomerulonephritis. In: Rudolph AM (ed).
Rudolph's Pediatrics, 20th edition. 1996, Stamford, CT: Appleton and Lang, pp.
1351-1366.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
Nephrotic syndrome:
A previously well 5 year old male presents to your office with the chief complaint of
facial puffiness. His mother noticed this a few days ago and it seems to be worsening.
He has no other symptoms, but about two weeks ago had "a bad cold."
Exam: VS T 37, HR 90, RR 20, BP 92/55. He is alert and cooperative with the
examination. His face shows moderate periorbital edema. His eyes are non-injected,
his conjunctiva are not edematous and his throat is not red. His heart is regular
without murmurs. Heart sounds are normal. His lung exam shows good aeration,
with no crackles or rhonchi. Abdomen is soft, non-tender, non-distended and without
masses or shifting dullness. No hepatosplenomegaly. He has normal male genitalia
with no scrotal edema. The dorsal surfaces of his hands and feet have mild pitting
edema. He has brisk capillary refill and 2+ pulses. No rashes are noted.
Urinalysis shows 4+ protein, and a specific gravity of 1.030. His chemistry panel is
remarkable for protein of 2 g/dL, serum albumin of 1.4 g/dL and cholesterol of 350
mg/dL. BUN and creatinine are normal.
He is not ill enough to require hospitalization. He is started on oral prednisone BID.
He is followed as an outpatient clinically and by daily urine dipsticks. His edema and
proteinuria gradually resolve with treatment. His corticosteroids are tapered off and
he remains stable.
Nephrotic syndrome describes the collection of clinical and laboratory findings
secondary to glomerular dysfunction, resulting in proteinuria. The diagnostic criteria
are marked proteinuria, generalized edema, hypoalbuminemia, and hyperlipidemia
(with hypercholesterolemia). The proteinuria in nephrotic syndrome is severe,
exceeding 50 mg of excreted protein for every kilogram of body weight over 24
hours. Primary nephrotic syndrome refers to diseases limited to the kidney, whereas
secondary nephrotic syndrome indicates systemic diseases that include kidney
involvement (e.g., diabetic nephropathy).
In healthy children (less than 18 years of age), the annual incidence of nephrotic
syndrome is 2-7 new cases per 100,000. The prevalence is approximately 16 cases
per 100,000 children, making nephrotic syndrome one of the most frequent reasons
for referral to a pediatric nephrologist. Also, the most common type of nephrotic
syndrome is recurrent to some degree, so cases will often manifest repeatedly over
time. The peak age for the onset of nephrotic syndrome is 2-3 years of age. In early
childhood, males outnumber females about 2:1 for new cases of nephrotic syndrome.
In adolescence and adults, the gender distribution is more equal. Primary nephrotic
syndrome is more common in children less than six years of age, while secondary
nephrotic syndrome predominates for patients older than six. The disease inheritance
is usually sporadic, although there is a congenital form of nephrotic syndrome, called
Finnish type congenital nephrosis, which is inherited in an autosomal recessive
manner. This abnormality has been mapped to a defect in the nephrin gene on
chromosome 19q13.1 that codes for a protein in the glomerular basement membrane.
The main pathogenic abnormality in nephrotic syndrome is an increase in glomerular
capillary wall permeability, resulting in pronounced proteinuria. The normal
glomerular wall is remarkably selective for retaining protein in the serum. Once this
selectivity is lost, the excretion of large amounts of protein will follow. This increase
in permeability is related to the loss of negatively charged glycoproteins within the
capillary wall that usually repel negatively charged proteins. The predominant protein
lost is albumin, although immunoglobulins are also excreted. The pathophysiology
for the formation of edema is incompletely understood. A simplification of the
predominant theory is that after the plasma albumin concentration drops, secondary to
protein excretion, the plasma oncotic pressure drops. With the decrease in oncotic
pressure, fluid moves from the intravascular space to the interstitial space causing
edema. The liver has a very large capacity to synthesize protein, so the persistent
hypoalbuminemia is likely not due entirely to increased losses. Reduction of the
intravascular volume results in activation of the renin-angiotensin-aldosterone system.
Sodium and water are retained, which further increases the edema. There are likely
other factors involved in the formation of edema, because some patients with
nephrotic syndrome have normal or increased intravascular volume.
The hyperlipidemia in nephrotic syndrome is characterized by elevated triglycerides
and cholesterol and is possibly secondary to two factors. The hypoproteinemia is
thought to stimulate protein synthesis in the liver, including the overproduction of
lipoproteins. Also lipid catabolism is decreased due to lower levels of lipoprotein
lipase, the main enzyme involved in lipoprotein breakdown.
More than 90% of children with primary nephrotic syndrome have idiopathic
nephrotic syndrome and this will be the focus of this chapter. The etiology of this
condition remains largely unknown, but some have postulated an immunologic
mechanism. Supporting evidence for this theory include the characteristic response to
corticosteroids and cytotoxic agents, an observed increased incidence of concurrent
allergic conditions, and spontaneous remissions with natural measles infections
(known to induce suppression of cell-mediated immunity). Evidence against an
immunologic etiology is a failure to identify immune reactants or inflammation in
kidney biopsies.
There are three morphological patterns of idiopathic nephrotic syndrome, with
minimal change disease (also called "nil disease") making up 80-85% of the cases. In
this condition, the glomeruli appear normal or have a minimal increase in the
mesangial cells or matrix. As well as being the most common form of primary
nephrotic syndrome, minimal change disease also has the mildest clinical course. The
rest of this chapter will focus on this disease entity after briefly describing the other
forms of primary nephrotic syndrome as well as secondary nephrotic syndrome. The
less commonly seen types of primary idiopathic nephrotic syndrome are focal
segmental
glomerular
sclerosis,
membranous
glomerulonephritis
and
membranoproliferative glomerulonephritis.
Focal segmental glomerular sclerosis is found in about 7-15% of patients with
nephrotic syndrome, making it the second most common primary renal lesion. It
tends to have a more severe clinical course with persistent proteinuria, progressive
decline in glomerular filtration rate and hypertension that can be unresponsive to
therapy. Renal failure occurs, with dialysis or transplant being the only treatment
options. Unfortunately, the recurrence rate of focal segmental glomerular sclerosis
can be as high as 40% after renal transplant.
Membranoproliferative glomerulonephritis accounts for roughly 7% of primary
idiopathic nephrotic syndrome. These patients often have hematuria, hypertension
and mild azotemia. Another characteristic finding is persistently depressed C3 levels.
The clinical course is variable with only a small percentage of patients going into
remission.
Membranous glomerulopathy is rare in the pediatric age group, but becomes more
common into adolescence and adulthood. It is often associated with infections, with
hepatitis B being the most common. The clinical course is variable, but the overall
prognosis is good, with spontaneous remission of proteinuria occurring in 50-60% of
cases.
There are many different causes of secondary nephrotic syndrome in children. These
include multisystemic diseases such as systemic lupus erythematosus and HenochSchonlein purpura, malignancies such as Hodgkin disease or leukemia, drug or toxin
exposures such as mercury, gold, penicillamine or bee sting, and infectious etiologies
such as Epstein-Barr virus, cytomegalovirus and tuberculosis.
Children with idiopathic nephrotic syndrome secondary to minimal change disease
usually present with edema. Clinically apparent edema usually is not seen until
albumin levels drop below 2 g/dL. The edema is initially noted around the eyes and
in the lower extremities. Over the course of a day, the edema often distributes from
the eyes to more dependent areas. After time, the edema becomes more pronounced,
generalizes and there can be weight gain. Patients or parents may notice tighter fit of
clothes, belts and shoes and scrotal or labial edema often occurs. As the edema
accumulates, pleural effusions, ascites and decreased urine output may develop. In
many cases, there is a history of preceding upper respiratory symptoms. Anorexia,
abdominal pain and diarrhea may be seen, possibly secondary to the formation of
ascites. Blood pressure and renal function are usually normal.
The hallmark of nephrotic syndrome is severe proteinuria, most reliably diagnosed
using a 24-hour urine collection. Spot urinalysis is also informative and reveals +3 to
+4 proteinuria (300 to 1000 mg/dL), with a specific gravity usually greater than
1.020. Gross hematuria is not common. Blood samples show decreased albumin
levels usually less than 2.0 mg/dL and elevated triglyceride and cholesterol levels.
Because of the hypoalbuminemia, hypocalcemia is often seen, with calcium levels
less than 9.0 mg/dL. Usually the ionized calcium will be normal. Hyponatremia and
hyperkalemia can be seen, with hyperkalemia developing in patients who are oliguric.
Serum C3 levels are normal in cases of minimal change disease.
Renal biopsy is not necessary for the child with newly diagnosed nephrotic syndrome
and the initial treatment will be the same, regardless of the cause. How the disease
responds to corticosteroids may help dictate the need for biopsy. If the response is
good and renal function is normal, the diagnosis of minimal change disease may be
presumed. If relapses respond to corticosteroids and there is no proteinuria during
disease free periods, this diagnosis is strengthened. Biopsy is generally obtained in
cases where there is poor or no response to corticosteroids, the patient is less than 1
year old (high likelihood of congenital nephrotic syndrome) or over 10 years old,
secondary nephrotic syndrome is suspected, there is corticosteroid toxicity, or the use
of a cytotoxic agent is being considered. Patients with low serum complement levels
or hypertension on presentation may require biopsy since these conditions are not
characteristic of minimal change disease and may indicate other renal lesions.
The treatment of primary idiopathic nephrotic syndrome of childhood is corticosteroid
therapy and supportive care. Steroid therapy will be discussed below. Many patients
may be treated on an outpatient basis, although the newly diagnosed patient is
sometimes admitted for diagnostic and educational purposes. Edema is managed with
sodium restriction (the "no added salt diet") and diuretics such as hydrochlorothiazide.
If hypokalemia develops, an oral potassium supplement or spironolactone may be
added. Aggressive use of loop diuretics may be harmful since most patients initially
presenting with nephrosis are hypovolemic. The use of diuretics necessitates close
monitoring of patients. Patients need to monitor their weight closely and consume
adequate amounts of protein.
Conditions that require immediate attention and hospitalization are severe scrotal
edema, dehydration (more than 10% dehydrated), respiratory compromise due to
pulmonary edema or pleural effusions, and peritonitis or suspected bacterial infection.
Despite their edematous appearance, most patients have decreased intravascular
volumes. Therapy is aimed at the restoration of intravascular volume and preventing
volume overload. Intravenous fluids are used, sometimes with the infusion of
albumin to increase the serum oncotic pressure. The albumin must be given slowly,
over 8-12 hours, to prevent fluid overload from rapid intravascular volume expansion.
There is some debate over the use of albumin, since the effect seems to be transient
and it is presumably excreted rapidly (1). Electrolyte levels and renal function must
be closely monitored. Once the intravascular volume is restored, diuretic therapy is
used to mobilize the fluid and prevent volume overload. Paracentesis is performed if
there is respiratory compromise secondary to severe ascites. Antibiotic therapy to
cover for the most common pathogens should be started if there is evidence of
bacterial infection (discussed below).
Minimal change disease is characteristically responsive to corticosteroid therapy and
once the diagnosis is confirmed with laboratory testing, steroid therapy should be
started. Prednisone is initiated with a dose of 60 mg/sq-meter/day or 2 mg/kg/day
divided in 2-3 doses. The daily dose is continued until the proteinuria resolves,
usually in 2-3 weeks. Some sources suggest continuing the daily dose for 4-6 weeks
(1). Regardless, the corticosteroids are continued and then tapered over the course of
3-6 months. In patients with minimal change nephrotic syndrome, approximately
98% will eventually have satisfactory therapeutic responses. This disease is one of
frequent relapse, with two thirds of patients having a single relapse and roughly one
third experiencing repeated relapses over many years. Most patients with steroidresponsive nephrotic syndrome will continue to have relapses until they are in their
late teens. Relapses are treated the same as the initial presentation. With repeated
relapses or severe steroid toxicity (growth retardation, elevated blood pressure),
cytotoxic agents such as cyclophosphamide are added to a lower corticosteroid dose.
This agent has been shown to prevent relapses and to increase the duration of
remission. Chlorambucil and less commonly cyclosporine have also been used for
remission induction. Another regimen for patients refractory to corticosteroids is
indomethacin and an angiotensin-converting enzyme (ACE) inhibitor.
The most common complications of nephrotic syndrome are bacterial infection and
thromboembolism. There are also complications secondary to medications such as
the gastric irritation and insulin resistance seen with corticosteroids or the
hemorrhagic cystitis, sterility and leukopenia seen with cyclophosphamide. The
tendency to develop infections, especially "primary peritonitis" (a type of
pneumococcal sepsis), is thought to be due to IgG excretion, decreased complement
function, and diminished splanchnic blood flow. The organisms causing peritonitis
are most commonly Streptococcus pneumoniae and Escherichia coli. Peritonitis
should always be considered in a patient who has nephrotic syndrome and abdominal
pain or fever. Antibiotics such as ampicillin or vancomycin with a third generation
cephalosporin or an aminoglycoside would provide good empiric coverage. Other
infections such as sepsis, cellulitis, pneumonia and urinary tract infection are also
seen. The signs of infection may be masked if the patient is currently on
corticosteroid therapy. Any child with nephrotic syndrome and a fever must be
thought of as having an infection until proven otherwise, since they are at high risk for
sepsis, similar to splenectomy patients. Because of their predilection for S.
pneumoniae infection, polyvalent pneumococcal vaccine should be administered to
children over two years of age.
Another complication, thromboembolism is thought to be more common secondary to
increased platelet aggregation, increased fibrinogen concentration, decreased
antithrombin III concentrations, increased blood viscosity and decreased blood flow.
Venous thrombosis is most common, especially in the renal vein, pulmonary artery,
and deep vessels of the extremities. In patients with refractory nephrosis, low dose
anticoagulants are sometimes used.
The prognosis for children with minimal change nephrotic syndrome is good, with
most patients ultimately becoming disease free and living a normal life. Mortality is
approximately 2% with the majority of deaths being secondary to complications such
as peritonitis or thromboembolic disease.
Questions
1. The most common cause of primary idiopathic nephrotic syndrome is:
a. Focal segmental glomerular sclerosis
b. Membranoproliferative glomerulonephritis
c. Membranous glomerulopathy
d. Minimal change disease
2. Common causes of mortality in primary nephrotic syndrome is/are:
a. Acute renal failure
b. Thromboembolism
c. Congestive heart failure
d. Peritonitis
e. Seizure
3. True/False: A renal biopsy is necessary to confirm the diagnosis of primary
idiopathic nephrotic syndrome.
4. The inheritance pattern of primary idiopathic nephrotic syndrome is/are:
a. Autosomal recessive
b. X-linked recessive
c. Autosomal dominant
d. Sporadic
5. Reasons for biopsy in a patient with nephrotic syndrome include:
a. Continued proteinuria after a week of prednisone therapy.
b. Age at onset of 10 months.
c. Relapse 1 year after initial course of therapy.
d. Cholesterol level greater than 400 mg/dL.
e. A patient who has a history of systemic lupus erythematosus
Answers to questions
1. d. Minimal change disease or "nil disease" accounts for 80-85% of cases of
primary idiopathic nephrotic syndrome in childhood.
2. b and d. Infection, especially peritonitis and thrombosis account for the
majority to nephrotic syndrome mortality.
3. false. The decision to perform a renal biopsy is usually deferred until the
initial course of corticosteroid is initiated, unless there are specific risk factors such as
age below one or above 10, hypertension on presentation or decreased complement on
presentation.
4. d. Primary nephrotic syndrome is sporadic in nature. Congenital nephrotic
syndrome is passed in an autosomal recessive manner.
5. b and e. Nephrotic syndrome in a child less than 1 year old may indicate congenital nephrotic syndrome and renal
biopsy is often performed. In a patient with SLE, the nephrotic syndrome is likely secondary and a renal biopsy is
indicatedReferences
1. Roth KS, Amaker BH, Chan JCM. Nephrotic Syndrome: Pathogenesis and
Management. Pediatr Rev 2002;23(7):237-248.
2. Bergstein JM. Chapter 535 - Nephrotic Syndrome. In: Berhman RE, et al
(eds). Nelson Textbook of Pediatrics, 16th edition. 2000, Philadelphia: WB
Saunders Co, pp. 1592-1595.
3. Chesney RW. The Idiopathic Nephrotic Syndrome. Curr Opin Pediatr
1999;11(2):158-161.
4. Penaflor G. Congenital Nephrotic Syndrome. Pediatr Rev 2001;22(1):32.
5. Orth SR, Ritz E. The Nephrotic Syndrome. New Engl J Med
1998;338(17):1202-1211.
6. Kate C, Norman ME. Nephrotic Syndrome. In: Fleisher GR, Ludwig S
(eds). Textbook of Pediatric Emergency Medicine, 4th edition. 2000, Baltimore:
Lippincott, William & Wilkins, pp. 832-835.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
Shock:
This 2 month old male infant with a 4 day history of vomiting and diarrhea is brought
to the emergency department by his mother. Initial findings in the emergency
department include:
Airway: Breath sounds are normal. Airway is patent.
Breathing: Breathing is regular at 45 breaths per minute, unlabored.
Circulation: Proximal pulses are poor, distal pulses are absent, and extremities are
cool. Feeling from the 5th toe upwards, the legs are cool up to the knee. Capillary
refill is 8 seconds. Heart rate is 209 beats per minute, and blood pressure is 70mmHg
systolic.
ECG: There are narrow QRS complexes with sinus tachycardia on the monitor.
The infant does not recognize his parents, is extremely lethargic, and responds to pain
only, with a minimal grimace.
You are unable to start an IV line, 100% oxygen is started. The mucous membranes
of the mouth are pink. An intraosseous (IO) is placed in the left tibia and 20cc/kg of
normal saline is infused as rapidly as possible. The infant is reassessed. Airway and
breathing remain stable. The heart rate is now 195. A repeat bolus of 20cc/kg is
given and the patient is reassessed. After the 3rd fluid bolus is given, the patient
becomes more alert, distal pulses return, and the patient improves throughout
resuscitation. The heart rate has come down to 160. However, a rapid bedside
glucose analysis reveals a blood sugar of only 32, which is quickly treated. This case
represents a patient in compensated hypovolemic shock (and hypoglycemia)
secondary to vomiting and diarrhea.
Shock is a clinical syndrome of circulatory dysfunction resulting in inadequate
oxygen and nutrient delivery, with inability to meet the metabolic demands of the
tissues (cells). This results in a cascade of events resulting in altered cellular
metabolism, function, structure, and ultimately death. Shock is NOT necessarily
hypotension. It begins with a normal blood pressure and progresses over time.
Normal circulatory function depends on 3 components: 1) adequate cardiac function
(the pump), 2) appropriate vascular tone (the pipes) and 3) adequate blood volume
(the fuel). When one of more of these circulatory components fail, shock results.
Adequate delivery of oxygen and nutrients is dependent on adequate cardiac output
(CO). Cardiac output in turn is dependent on two cardiac factors: 1) heart rate (HR)
and 2) stroke volume (SV). As children are "heart rate dependent", the heart rate is
the single most important vital sign when determining shock. The heart rate itself is
regulated by two factors: 1) vagal tone and 2) catecholamines. Catecholamines are
released in response to stress and have two major circulatory effects in children: 1)
increase in heart rate (i.e., tachycardia), and 2) increase in peripheral vascular tone
(resistance) (i.e., vasoconstriction, resulting in cool, clamped down extremities).
Stroke volume is the second determinant of cardiac output, and is dependent on three
factors: 1) preload (intravascular volume/blood often called "venous return"), (the
fuel), 2) myocardial contractility (heart muscle function), (the pump), and 3) afterload
(systemic vascular resistance) (the pipes). Children are particularly dependent upon
adequate intravascular volume, and when volume depleted, they peripherally
vasoconstrict to maintain stroke volume. The myocardium in infants is "stiff" and
plays little role in increasing cardiac output. Therefore, the heart rate must increase in
order to maintain adequate circulatory function. Remember C.O.=H.R. x S.V.
Shock is a dynamic process that if untreated, progresses through three phases: 1)
compensated, then 2) uncompensated, and finally 3) irreversible. Compensated
shock, by definition, occurs in a body, which has successfully compensated to a
circulatory disruption and is maintaining adequate vital organ perfusion and
oxygenation. This may be difficult to differentiate from the patient's normal status.
Blood pressure is normal. Tachycardia is usually present, and as catecholamine
release increases, the heart rate increases and peripheral vasoconstriction with
prolonged (delayed) capillary refill occurs. Utilizing my exam technique, "The
pediatrician's handshake", I first feel the 5th toe. If the 5th toe is cold with a
prolonged capillary refill, I progress to the other toes, up the foot, then the leg. The
further up the leg the capillary refill is prolonged and the leg(s) is cool, the more
vasoconstricted the body is, and when counted, the faster the heart rate will be.
Normal capillary refill is 2 seconds or less, about the time I take to say "pepperoni
pizza". If I need to add "more toppings" to my pizza, then I know the capillary refill
is prolonged and the body is "in shock". There are pitfalls when interpreting capillary
refill; if the body is developing a fever, or in a cold environment, vasoconstriction
results and capillary refill is not a reliable sign of shock. Like any other single sign,
this must be taken in context with all other findings.
As shock progresses (hopefully by now you will have intervened and are reversing
and treating the cause and it will not progress), the compensatory mechanisms (i.e.,
increasing heart rate and vasoconstriction) reach their maximum ability and cannot
increase further, then suddenly the body decompensates. Metabolic demands are not
met, and cellular ischemia results in the release of vasoactive mediators which affect
the microcirculation resulting in end-organ compromise and acidosis, with signs
including hypotension, altered mentation, oliguria, acidosis, mottled pale skin with
cool extremities, tachypnea and dyspnea, tachycardia and the obvious appearance of
an "sick body". At this point irreversible damage of key organs (heart, brain, kidneys)
may have occurred, but aggressive therapy is still indicated in chance that
cardiovascular measurements can still be normalized. Unfortunately, despite
aggressive therapy, death may occur regardless of therapy.
The main point to reemphasize is: the early recognition and treatment of
compensated shock (better prognosis) is essential to prevent decompensated and
irreversible shock (poor prognosis, high risk of death). Remember, hypotension is a
late sign of shock and should not be allowed to occur.
Important historical information and physical exam findings must be included when
considering the clinical manifestations and differential diagnosis of shock. Historical
information asked must include: 1) age, 2) preexisting conditions/illness, 3) fever, 4)
vomiting/diarrhea, 5) poor feeding, 6) urine output, 7) lethargy, 8) trauma, 9) toxic
ingestion. The physical exam must include: 1) general appearance/alertness/eye
contact/activity, 2) heart rate, 3) skin perfusion, a) capillary refill, b) color, c) skin
temperature, 4) oliguria (if an observation period is permitted), 5) altered mental
status, 6) tachypnea, 7) fever, 8) blood pressure, to name a few.
Utilizing history and physical exam information, it is important to classify the shock
syndrome into one of 3 major etiologies: 1) hypovolemic shock, a) absolute, b)
relative, 2) septic shock, or 3) cardiogenic shock. Hypovolemic shock is the most
common cause of shock in children. A loss of circulating blood volume results in
decreased preload (the fuel) with resultant decreased cardiac output. Absolute
hypovolemia has three major causes; 1) dehydration secondary to a) diarrhea and
vomiting or b) poor intake; 2) hemorrhage or 3) renal losses of fluid from a) diabetes
mellitus or b) diabetes insipidus.
"Relative" hypovolemic shock is also called "distributive shock" where the problem is
not related to absolute volume but to loss of vessel tone resulting in vasodilation and
therefore a larger intravascular space (secondary to vasodilation) with a "normal
blood volume", which results in a "relative" hypovolemia. There are four major
causes of this: 1) sepsis, 2) anaphylaxis, 3) spinal cord injury or 4) drug reactions
secondary to drugs such as barbiturates, phenothiazines, and antihypertensives.
Septic shock is a microcirculatory dysfunction that results from activation of a
systemic inflammatory response from age-group specific bacterial pathogens. Those
at risk for septic shock include: oncology patients, those with central venous
(oncology) catheters, on chronic high dose steroids, or with a congenital or acquired
immunodeficiency. Gram negative bacterial endotoxin-mediated septic shock results
in activation of numerous mediators of circulatory failure. The end result is impaired
myocardial contractility, alteration in vascular tone, and capillary leak.
Lastly, cardiogenic shock (the pump) may be the primary cause of shock or a late
manifestation of other forms of shock. Here, there is an abnormality in cardiac
function due to depressed myocardial contractility. Etiologies include 1) congenital
heart disease, 2) myocardial infarction (e.g., Kawasaki's), 3) myocarditis or
pericarditis, 4) congestive heart failure, 5) cardiac trauma, 6) dysrhythmia, or 7) drugs
affecting myocardial contractility.
The table below compares the cardiac output (CO), systemic vascular resistance
(SVR) and central venous pressure (CVP) in the three shock syndromes. An increase
in CVP will clinically be seen as distended neck veins and an enlarged liver. An
increase in SVR will be seen as cold, clamped down extremities with weak pulses and
prolonged capillary refill.
Hypovolemic
Cardiogenic
Distributive
CO
decreased
decreased
increased
SVR
increased
increased
decreased
CVP
decreased
increased
decreased
Common Etiologies of Shock Syndromes
1) Hypovolemic Shock
a) Absolute Hypovolemia: water and electrolyte losses (diarrhea, vomiting,
diabetes insipidus, renal losses, heat stroke, intestinal obstruction, burns), hemorrhage
(trauma, surgery, GI bleeding), plasma losses (burns, nephrotic syndrome, sepsis,
intestinal obstruction, peritonitis).
b) Relative Hypovolemia (distributive shock): anaphylaxis (antibiotics, blood
products, insects, vaccines, local anesthetics, foods, etc.), neurologic injury (head
injury, spinal shock), drug intoxication (barbiturates, phenothiazines, tranquilizers,
antihypertensives), sepsis., toxic shock
2) Septic Shock
a) Bacterial: Group A streptococcus, Haemophilus influenzae type b,
Neisseria meningitidis, Streptococcus pneumoniae, Group B Streptococcus, Gram
negative bacilli (predominantly E. coli), Staphylococcus aureus.
b) Other: viral, fungal, rickettsial
3) Cardiogenic Shock:
Congenital heart disease (ductal-dependent systemic blood flow, postoperative complications), dysrhythmias, drug intoxication, myocarditis (viral, other
inflammatory), hypoxic-ischemic injury (Kawasaki syndrome, perinatal asphyxia,
near-drowning, near-SIDS), primary cardiomyopathy, metabolic derangement
(hypoglycemia, acidosis), hypothermia, late sepsis, toxic shock.
4) Miscellaneous Shock Syndromes
a) Obstructive Shock:
pericardial tamponade, tension pneumothorax,
pulmonary embolus
b) Dissociative Shock: carbon monoxide poisoning, methemoglobinemia
Regardless of etiology, initial therapy is universal. All patients should receive
100% supplemental oxygen by face mask, followed by the correction of the mismatch
between metabolic supply and demand. Early recognition and aggressive treatment is
the key. Anticipation of the effects of shock as a dynamic, clinical syndrome with
multi-system consequences, which can be reversed, with optimization of cardiac
output is essential to prevent decompensation and irreversible shock.
Treatment can be classified broadly into: 1) oxygenation, 2) vascular access, 3) fluid
administration, and 4) drug therapy. Oxygenation includes providing 100% oxygen
and also assuring adequate hemoglobin, stopping hemorrhage, and replacing blood if
the hematocrit is less than 30%. Consider endotracheal intubation, but be aware of
the cardiovascular effects that intubation and positive ventilation can cause, such as
bradycardia, hypotension or reduced venous return. Vascular access includes
insertion of a (preferably two) large intravenous catheters, and obtaining necessary lab
tests (CBC, blood culture, electrolytes, BUN, creatinine, glucose, calcium,
coagulation profile and blood gas). If vascular access is difficult to obtain, use an
intraosseous (IO) device and insert this into the tibia. New guidelines allow IO use in
children of all ages. There are 2 major types of fluid that can be administered,
crystalloid or colloid. Crystalloid is either volume expanding isotonic (normal saline
or Ringer's lactate) or hypertonic (3% saline). Crystalloid is an effective volume
expander in resuscitation but requires 2-4 times the volume of blood loss to restore
hemodynamic parameters. Of the isotonic volume infused into the extracellular
compartment (i.e., intravenous or I.O.), only 25% remains intravascular, while 75%
eventually goes interstitial. This has led to the use of hypertonic saline (3% sodium
chloride solution) in certain situations (such as hemorrhagic shock), as it is an
effective vascular volume expander using less volume, working by expanding the
ECF by a greater amount than the volume infused because it pulls water from the ICF
compartment. Although there is less potential for edema as a result of its use, there
are complications including increased serum osmolarity, increased serum Na and C1
levels, metabolic acidosis, and cerebral dehydration and hemorrhage.
Colloid refers to 5% albumin, fresh frozen plasma (FFP) or blood. Albumin's major
advantage is that it remains primarily within the intravascular space (less enters the
interstitial space). In addition, it can draw extravascular water into the intravascular
space because of its oncotic pressure effect. FFP is an effective volume expander
with the added benefit of procoagulant factors, while blood (whole or packed RBCs)
is an effective volume expander with the added benefit of oxygen carrying capacity.
Most often fluid administration in the form of volume resuscitation is accomplished
by the infusion of 0.9% sodium chloride (normal saline) or Ringer's lactate 20 ml/kg
IV bolus as quickly as possible. Then reevaluate and repeat the bolus depending on
the clinical status/changes. Search for other causes such as sepsis or occult
hemorrhage. Central venous pressure monitoring will help fluid management in
critical patients. General guidelines are to be liberal and aggressive with fluid
resuscitation, giving 20 ml/kg initially and repeating as needed. For septic shock,
more than 40ml/kg in the first hour has been shown to improve outcome. When
approaching 80 ml/kg, consider the use of an inotropic agent such as dopamine or
epinephrine.
Pharmacologic support includes medications that: 1) augment cardiac contractility
(inotropic/cardiotonic), vasoconstrictors to reverse inappropriate vasodilation, and
sometimes vasodilator drugs to reduce preload and afterload in cardiogenic etiologies,
2) antibiotics (for septic shock), 3) sodium bicarbonate, 4) calcium, 5)
immunotherapies, and 6) controversial therapy. However, before specific drugs are
described, a review of adrenergic receptor physiology is indicated. Each receptor has
a different physiologic response, as noted here:
Alpha:
Arteriolar constriction
Beta-1:
Increased myocardial contractility (inotropy)
Increased heart rate (chronotropy)
Beta-2:
Peripheral vasodilation
Bronchial smooth muscle relaxation
Dopaminergic:Smooth muscle relaxation
Increase renal blood flow
Examples of classic agonists include phenylephrine (pure alpha), isoproterenol (pure
beta, both beta-1 and beta-2), dobutamine (selective beta-1), albuterol (selective beta2), epinephrine (both alpha and beta).
Three commonly used inotropic drugs include dopamine, dobutamine and
epinephrine. Dopamine effects are dependent on the dose infused. Low dose (1-2
mcg/kg/min) results in vasodilation of the splanchnic (renal) and cerebral vascular
beds. Mid-dose (3-10 mcg/kg/min) has primarily a beta effect (chronotropic and
inotropic), while a higher dose (> 10 mcg/kg/min) has a pure alpha effect (pressor).
Dobutamine has a pure beta-1 (chronotropic and inotropic) effect, the effective dose
used ranging from 2-20 mcg/kg/min or greater. Epinephrine at an infusion dose of
0.05-2 mcg/kg/min has both beta and alpha effects, and may cause severe peripheral
vasoconstriction or arrhythmias.
Examples of vasodilator drugs used for "afterload reduction" in a failing heart to ease
the work of "pumping" are nitrates such as nitroprusside and nitroglycerine.
Nitroprusside, infused continuously at a rate between 1-10 mcg/kg/min, is a
vasodilator working on both resistance and capacitance sides of the circulation;
however with time, a toxic cyanide metabolite is formed.
Consider the use of sodium bicarbonate after assuring adequate volume resuscitation
and ventilation, at a dose of 1-2 mEq/kg.
Calcium: Hypocalcemia can occur after tissue hypoperfusion of any etiology and can
result in myocardial depression and hypotension. If hypocalcemia is documented in a
symptomatic patient not responding to inotropes and pressors, then consider treating
the hypocalcemia. The dose used is 10-20 mg/kg CaCl (0.1-0.2 ml/kg of 10%
solution). Monitor ionized calcium levels to determine its need.
Antibiotics are used for septic shock (or presumed septic shock). For ages less than 6
weeks, a combination of ampicillin plus cefotaxime can be used. For ages greater
than 6 weeks cefotaxime or ceftriaxone can be used.
Immunotherapies include the use of anti-endotoxin (HA-1A or E5), anti-tumor
necrosis factor (TNFa) and interleukin-1 (IL-1) receptor antagonist. These are beyond
the scope of this chapter, but will be important adjuncts to antibiotics and intensive
care treatment in the future. Controversial therapy includes primarily the use of
steroids. Although theoretically it may be of benefit modulating the immune response
to sepsis, there has been no benefit (actually increased mortality) in humans, therefore
it is NOT currently recommended. A more detailed description of additional
medications utilized for resuscitation can be found in the chapter on pediatric
pulmocardiac resuscitation.
Ongoing assessment of the patient in shock includes repeated reassessments of the
physical exam, and monitoring equipment including pulse oximetry, cardiorespiratory
monitoring, repeated blood pressures, central venous pressure (if indicated), and urine
output through a catheter. Physical exam findings will be reflected in signs of
improved perfusion which will include improvement in appearance, including
alertness (mental status), eye contact, skin capillary refill, color and temperature, heart
rate and pulse strength, urine output, respiratory pattern and rate, and blood pressure.
Resolving metabolic acidosis and declining serum lactate levels are lab findings
indicating improvement of perfusion.
In summary, shock is a clinical syndrome NOT defined by blood pressure alone.
Worldwide, hypovolemic shock from diarrhea represents the leading cause of death.
Normal circulatory function depends on three factors: cardiac function (the pump),
vascular tone (the pipes), and blood volume (the fuel). A disturbance in one or more,
resulting in inadequate delivery of oxygen and nutrients to the tissues, leads to shock.
Shock is a progressive, dynamic process where early recognition and immediate
management (initially in the form of IV fluids) is essential to prevent deterioration
into decompensated and finally irreversible shock.
Questions
1. Prioritize the initial management of the child with shock:
a. Administer oxygen
b. Administer volume resuscitation
c. Support a patent airway
d. Support blood pressure and perfusion with cardioactive drugs
e. Administer antibiotics
f. Address oxygen carrying capacity with administration of blood if anemia is
present
2. The most sensitive indicator of intravascular volume in the pediatric patient is:
a. Cardiac output
b. Preload
c. Heart rate
d. Stroke volume
3. In the trauma patient with compensated shock, who is otherwise stable blood
should be considered as part of volume resuscitation:
a. Immediately after the airway is secured and intravenous access
b. After 20 cc/kg of isotonic fluid has been administered without clinical
response
c. After 40 cc/kg of isotonic fluid has been administered without clinical
response
d. After 60 cc/kg of isotonic fluid has been administered without clinical
response
e. After isotonic fluid administration has resulted in inadequate clinical
response and the patient requires operative repair
4. Which circulatory finding is the hallmark of the diagnosis of late (decompensated)
shock?
a. Capillary refill of 4 seconds
b. Altered mental status
c. Depressed anterior fontanelle
d. Hypotension
e. Absent distal pulses
5. An alert, 6 month old male has a history of vomiting and diarrhea. He appears
pale and has an RR of 45 breaths per minute, HR of 180 beats per minute, and a
systolic blood pressure of 85 mm Hg. His extremities are cool and mottled with a
capillary refill time of 4 seconds. What would best describe his circulatory status?
a. Normal circulatory status
b. Early (compensated) shock caused by hypovolemia
c. Early (compensated)shock caused by supraventricular tachycardia
d. Late (decompensated) shock caused by hypovolemia
e. Late (decompensated) shock caused by supraventricular tachycardia
6. Appropriate initial management for the child described in question 6 would include
which of the following?
a. Initiation of oral rehydration therapy
b. Placement of an intraosseous line, fluid bolus of 20 ml/kg of normal saline
c. Placement of an intravenous (IV) line, fluid bolus of 20 ml/kg of normal
saline
d. Placement of an IV line, adenosine 0.1 mg/kg IV
7. A 2 month old infant is brought to the ED with a pulse of 180 and BP 50/35 mm
Hg. A liver edge is palpable to the umbilicus. Skin is mottled, capillary refill is 6
seconds with weak distal pulses. Chest x-ray reveals cardiomegaly. During the
administration of 20 ml/kg of Ringer's lactate, respirations become labored and rales
are heard. The next step would be:
a. Sodium bicarbonate 1 mEq/kg IV
b. Repeat fluid bolus 20 ml/kg
c. Dopamine 5 to 10 mcg/kg/min IV infusion
d. Synchronous cardioversion 0.5 joule/kg
e. Epinephrine 0.01 mg/kg of the 1:10,000 solution IV
Answers to questions
1. c,a,b,d,f,e
2. c
3. c
4. d
5. b
6. c
7. c. This represents a case of cardiomyopathy with four classic findings of
congestive heart failure. Note that the patient's condition worsened with fluid
administration. Dopamine would be the first agent to try. Epinephrine may be used
later in desperation since its alpha effect may have detrimental consequences on
overall circulation.
References
1. AAP, ACEP. APLS: The Pediatric Emergency Medicine Course, Third
Edition, 1998.
2. Atici A, Satar M, Alparslan N. Serum interleukin-1 beta in neonatal sepsis.
Acta Pediatr 1996;85:371-374.
3. Bell LM. Shock. In: Fleisher GR, Ludwig S (eds). Textbook of Pediatric
Emergency Medicine, 4th edition. 2000, Baltimore: Lippincott, Williams and
Wilkins, pp. 47-57.
4. Bone RC. A critical evaluation of new agents for the treatment of sepsis.
JAMA 1991;266(12):1686-1691.
5. Cardillo JA, Davis AL, Zaritsky A. Role of early fluid resuscitation in
pediatric septic shock. JAMA 1991;266:1242-1245.
6. Bhende MS, Thompson AE, Orr RA. Utility of an end-tidal carbon dioxide
detector during stabilization and transport of critically ill children. Pediatrics
1992;89-1042-1044.
7. Griffel MI, Kaufman BS. Pharmacology of colloids and crystalloids. Crit
Care Clin 1992;8(2):235-253.
8. Velasco AL, et al. Intraosseous infusion of fluids in the initial management
of hypovolemic shock in young subjects. J Pediatr Surg 1991;26(1):4-8.
9. Tobin JR, Wetzel RC. Shock and Multi-Organ System Dysfunction. In:
Rogers MC, Nichols DG (eds). Textbook of Pediatric Intensive Care, Williams and
Wilkins, Philadelphia 1996, pp. 555-605.
10. Witte MK, Hill JH, Blumer JL. Shock in the Pediatric Patient. Adv
Pediatr 1987;34:139173.
.
Jordan University of Science and Technology
Faculty of Medicine
Department of Pediatrics
Case No.
FTT:
This is a 12 month old female who presents for a well child check. Within the past 4
months, her weight has fallen from the 25th percentile to significantly less than the
5th percentile. Her height has dropped from the 10th percentile to slightly less than
the 5th percentile, while her head circumference has remained at about the 25th
percentile. Her language, motor, cognitive, and social development are normal. She
seems to eat appropriate foods for her age, but the her mother notes that she tends to
be restless and fidgety while eating, and that she does not like the texture of certain
foods, often leading to parental frustration at mealtimes. Her stools tend to be
frequent, with particles of food seen. Urine is normal. There are no symptoms of
respiratory or neurological disease, and her review of systems is otherwise negative.
Her past medical history is entirely unremarkable. She was born at term, weighing
3.0 kg (6 pounds, 10 ounces), without any perinatal complications. Her family history
is negative for any endocrinopathies or chronic illnesses. Mother is 155 cm (5 feet, 1
inch) 61 inches tall, and father is 168 cm (5 feet, 6 inches). Mother experienced
menarche at age 12.5 years and recalls that there were other children in the family
who were deemed small as young children but who caught up later in childhood.
Mother describes a history of increased sadness and worry since her child was born.
Parents are married, and there is no history of abuse or violence in the household.
Exam: Vital signs, including blood pressure, are normal. Weight 7 kg (< 5th
percentile), height 70 cm (5th percentile), head circumference 45.5 cm (50th
percentile). She is alert and interactive, and appears to relate well with her mother.
Her anterior fontanelle is still open, roughly 2 cm. Two teeth (one just emerging) are
present. Thyroid, lymph nodes, heart, lungs, abdomen, genitalia, nervous system, and
skin are all normal.
Laboratory studies: CBC, chemistry panel, lead level, TSH, urinalysis, PPD, and
stool studies, including ova and parasites all normal. Bone age is consistent with
skeletal maturity of an 8 month old infant.
Formulation: Failure to thrive, with components of genetic short stature with a family
history of constitutional growth delay. Parental anxiety, active temperament, and
oral/tactile sensitivity may lead to feeding difficulties.
Clinical course: The primary care physician sees the child and family every few
months for support, ongoing education, and coordination of services. A dietitian
assists in devising an enhanced calorie diet for the child. An occupational therapist
offers suggestions for reducing sensitivity to certain food textures. A psychiatrist
provides treatment for what is felt to be mild post-partum depression in the mother.
Two years later, the child is at the 5th percentile for both height and weight and is
otherwise doing well.
Failure to thrive (FTT) is a clinical sign (rather than a specific diagnosis) that
describes inadequate growth and is defined as (1) a child younger than 2 years of age
whose: 1) weight is below the 3rd or 5th percentile for age on more than one
determination; 2) weight is less than 80% of ideal weight (i.e., 0.8 times ideal weight)
for age; or 3) weight crosses two major percentile curves on a standardized growth
grid (2). FTT is a relatively common problem that may be seen in 10% of children in
a rural primary care setting during the first year of life and may account for 1% to 5%
of all referrals to children's hospitals and tertiary care settings (3).
The etiologies of FTT are diverse. To arrive at a list of differential diagnoses (which
essentially can include most pediatric illnesses), one can think critically about the
disease conditions that may interfere with any of the steps in the mechanisms leading
to normal growth.
Feeding etiologies include food unavailability, neglect, improper feeding technique,
appetite loss, food refusal, and neurologic conditions with impaired swallowing.
Other etiologies may include intestinal disease conditions or anatomic abnormalities,
endocrinopathies, chronic or prolonged infection, malignancies, other chronic
diseases, toxic substances and metabolic derangements.
The patterns of increase in weight, height, and head circumference over time are
important to note. Weight reduction out of proportion to height and head
circumference may result from malnutrition. Weight reduction in proportion to height
reduction but with sparing of head circumference may result from structural
dystrophies, genetic short stature, and other endocrinopathies. Global weight, height,
and head circumference reduction may result from central nervous system defects or
intrauterine growth retardation. The timing of the reduction in weight may also be
important to note, as a fairly sudden reduction in velocity of weight gain may reflect
either the development of a chronic illness or an adverse social circumstance. Other
specific signs and symptoms associated with FTT will vary according to the specific
etiology (4).
Because the differential diagnosis can be so broad, a thorough history, growth chart
review, and physical examination are key in the evaluation of FTT. Mindful of the
mechanisms underlying normal and abnormal growth, one should elicit: a thorough
feeding history (beyond the usual "diet" history), a comprehensive review of systems
(which often gets dismissed in favor of "as per HPI"), a developmental and
psychosocial history, and a history of family members' growth, development, and
medical illnesses. It is potentially useful to determine the mid-parental height. For
boys: father's height + mother's height + 13 cm]/2. For girls: father's height - 13 cm
+ mother's height]/2. Plot this on the growth grid for 2 to 18 year olds, estimate the
percentile of the child's predicted height, and compare this to the current growth
percentiles (as was done in the case above). However, parental short stature does not
automatically imply genetic short stature, as either of the parents could have been
adversely affected by malnutrition and/or chronic illness. Physical exam should
include all vital signs and organ systems and should note the child's level of physical
development (e.g., fontanelle size, dentition, etc.).
In the child with a "normal" history and physical examination, there is no clear
consensus on what should constitute a "screening" laboratory evaluation, and it is
possible that certain laboratory tests may have more value for reassurance rather than
substantial diagnostic yield. However, it may be reasonable to consider a complete
blood count, basic chemistries, urinalysis, and tuberculin test (1). Laboratory tests
that would be appropriate for routine well child care (e.g., lead level) are also
reasonable. Further laboratory tests should be guided by specific findings on history
and physical examination. In the case above, stool studies were obtained because of
the possibly abnormal stools, and a TSH and bone age were obtained because of the
possibly delayed skeletal growth.
Management of FTT is based upon a sound understanding of the underlying
etiologies. Traditionally, FTT has been categorized into "organic" and "non-organic"
etiologies, but recently, it has been recognized that nearly every case of FTT may
have both "organic" components (e.g., cyanotic heart disease) and "non-organic"
components (e.g., the stressful feeding interactions taking place around a fragile,
fussy child), thereby challenging the clinician to approach management in a
comprehensive manner. For the case described above, there are multiple interactions
between the various factors: for example, the perception of an "abnormal" child
(being small for age; albeit with a strong constitutional element) could increase
parental anxiety (compounding the depressive symptoms) and could render the
parent-child interactions (already challenged by the child's active temperament and
sensitivity to food textures) even more difficult. Hence, ongoing support and
education from the primary care physician and referrals to psychiatry and
occupational therapy become important components of the treatment plan.
An important priority is the establishment and maintenance of adequate nutrition, and
caloric intake should be determined based upon child's age, need for "catch up"
growth, and (if applicable) increased needs in illness. Creative methods, including
more frequent meals and snacks, high calorie foods (e.g., peanut butter, margarine),
and nutritional supplementation (e.g., PediaSure) can be employed to augment the
child's natural intake. If these methods are unsuccessful after a reasonable trial,
supplementation via nasogastric feeds may be indicated.
In the past, hospitalization was felt to be important in differentiating between
"organic" and "non-organic" etiologies, as it was believed that the former would not
gain weight in the hospital, while the latter would. However, hospitalization may not
necessarily distinguish the two, as children with "organic" FTT may, in fact, gain
weight with around the clock feeding in the hospital, while certain children with "nonorganic" FTT (e.g., with conditions involving an anxious temperament and/or
difficulty adjusting to new environments) may become even less likely to feed in a
hospital setting. On the other hand, hospitalization must be considered in cases where
a child is at risk of serious medical morbidity as a result of either malnutrition or the
condition underlying the FTT or at risk of neglect or abuse.
Children with FTT have been found to be at higher risk for adverse cognitive and
other developmental/behavioral outcomes, although it is not clear the extent to which
these are related to the medical conditions underlying the FTT, subtle neurological
dysfunctions which manifested themselves pre-morbidly as feeding and interactional
difficulties (hence FTT), or ongoing psychosocial adversity. Nevertheless, because of
the known importance of good nutrition for brain development during the first few
years of life, it is highly important to identify any potential growth disturbances and
suboptimal feeding practices during routine well child care and to expeditiously
manage FTT.
Questions (true or false):
1. "Organic" and "non-organic" FTT are clearly defined conditions which
enable pediatricians to focus treatment on "organic" cases.
2. Hospitalization is indicated when a child is at risk of serious medical
morbidity or abuse/neglect.
3. In addition, all children with FTT should be hospitalized to distinguish
between "organic" and "non-organic" etiologies.
4. Blood pressure is useful in evaluating young children with FTT.
5. If both parents are of short stature, then the child must have genetic short
stature.
6. History, growth chart review, and physical are key in the evaluation of
FTT.
7. In evaluating a child with FTT, it may be important to elicit any history of
excessive thirst, increased urination, and family members with renal disease.
Answers to questions
1.F, 2.T, 3.F, 4.T (can help detect renal disorders), 5.F, 6.T, 7.T
References
1. Zenel JA. Failure To Thrive: A General Pediatrician's Perspective.
Pediatr Rev 1997;18: 371-378.
2. http://www.cdc.gov/growthcharts
3. Kirkland RT. Failure to Thrive. In: McMillan JA, DeAngelis CD, Feigin
RD, Warshaw JB (eds). Oski's Textbook of Pediatrics, third edition. 1999,
Philadelphia: Lippincott Williams & Wilkins, pp. 752-755.
4. Gotlin RW, Klingensmith GJ. Chapter 26 - Endocrine Disorders. In:
Hathaway WE, Groothuis JR, Hay WW, Paisley JW (eds). Current Pediatric
Diagnosis and Treatment, 9th edition. 1991, Englewood Cliffs, NJ: Appleton &
Lange, pp. 773-774.