* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download AP Lab #10: Preparation of Ester
Bottromycin wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Ene reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
VX (nerve agent) wikipedia , lookup
Sulfuric acid wikipedia , lookup
Hydroformylation wikipedia , lookup
Butyric acid wikipedia , lookup
Petasis reaction wikipedia , lookup
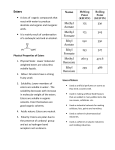
10 The Preparation and Properties of Esters Objective Esters are an important class of organic chemical compounds. The bonding in esters is analogous to that found in some important classes of biological compounds. Several esters will be prepared in this experiment to illustrate the most common methods of synthesis; some of the esters have characteristic odors that you will most likely recognize. General Introduction to Esters When an organic acid, R-COOH, is heated with an alcohol, R'-OH, in the presence of a strong mineral acid, the chief organic product is a member of the family of organic compounds known as esters. The general reaction for the esterification of an organic acid with an alcohol is R-COOH + HO-R' R-CO-OR' + H20 In this general reaction, R and R' represent hydrocarbon chains, which may be the same or different. As a specific example, suppose acetic acid, CH3COOH, is heated with ethyl alcohol, CH 3CH20H, in the presence of a mineral acid catalyst. The esterification reaction will be CH3-COOH + HO-CH2CH3 CH3-COO-CH2CH3 + H20 The ester product of this reaction (CH3-COO-CH2CH3) is named ethyl acetate, indicating the acid and alcohol from which it is prepared. Esterification is an equilibrium reaction, which means that the reaction does not go to completion on its own. Frequently, however, the esters produced are extremely volatile and can be removed from the system by distillation. If the ester is not very easily distilled, it may be possible instead to add a desiccant to the equilibrium system, thereby removing water from the system and forcing the equilibrium to the right. Introduction The general reaction for the esterification of an organic acid with an alcohol is R-COOH + HO-R' R-CO-OR' + H20 In this general reaction, Rand R' represent hydrocarbon chains, which may be the same or different. Unlike many organic chemical compounds, esters often have very pleasant, fruitlike odors. Many of the odors and flavorings of fruits and flowers are due to the presence of esters in the essential oils of these materials. The table that follows lists some esters with pleasant fragrances, as well as indicating from what alcohol and which acid the ester may be prepared. A fruit or flower generally contains only a few drops of ester, giving a very subtle odor. Usually, the ester is part of some complex mixture of substances, which, taken as a whole, have the aroma attributed to the material. When prepared in the laboratory in relatively large amounts, the ester may seem to have a pronounced chemical odor, and it may be difficult to recognize the fruit or flower that has this aroma. Ester n-propyl acetate methyl butyrate isobutyl propionate octyl acetate methyl anthranilate isoamyl acetate ethyl butyrate benzyl acetate Table of Common Esters Aroma Constituents pears n-propyl alcohol/acetic acid apples methyl alcohollbutyric acid rum isobutyl alcohol/propionic acid oranges n-octyl alcohol/acetic acid grapes methyl alcohol/2-aminobenzoic acid bananas isoamyl alcohol/acetic acid pineapples ethyl alcohollbutyric acid peaches benzyl alcohol/acetic acid Exp 10: The Preparation and Properties of Esters -2- Apparatus/Reagents Required Hotplate; 50% sulfuric acid; assorted alcohols and organic acids, as provided by the instructor, for the preparation of fruit and flower aromas; methyl salicylate; 20% NaOH; disposable 4 mL plastic pipets with stem cut to 2.5 cm. Safety Precautions Protective eyewear approved by your institution must be worn at all times while you are in the laboratory. Most of the organic compounds used or produced in this experiment are highly flammable. All heating will be done using a hotplate, and no flames will be permitted in the laboratory. Sulfuric acid is used as a catalyst for the esterification reactions. Sulfuric acid is dangerous and can bum skin very badly. If it is spilled, wash immediately before the acid has a chance to caus.e a bum, and inform the instructor. The vapors of the esters produced in this experiment may be harmful. When determining the odors of the esters produced in this experiment, do not deeply inhale the vapors. Merely waft a small amount of vapor from the ester toward your nose. NaOH solution is highly corrosive to eyes and skin. Wash immediately if spilled. Procedure Set up a water bath in a 250-mL beaker on a hotplate in the exhaust hood. Most of the reactants and products in this choice are highly flammable, and no flames are permitted in the lab during this experiment. Adjust the heating control to maintain a temperature of around 70°C in the water bath. Some common esters, and the acids/alcohols from which they are synthesized, were indicated in the table in the Introduction to this choice. Synthesize at least two of the esters, and note their aromas. Different students might synthesize different esters, as directed by the instructor, and compare the odors of the products. To synthesize the esters, mix 3-4 drops (or approximately 0.1 g if the acid is a solid) of the appropriate acid with 3-4 drops of the indicated alcohol on a clean, dry watch glass. Add 1 drop of 50% sulfuric acid to the mixture on the watch glass (Caution!). Use the tip of a plastic pipet to stir the mixture on the watch glass, and then suck as much as possible of the mixture into the pipet. Place the pipet, tip upward, into the warm-water bath, and allow it to heat for approximately 5 minutes. Squirt the resulting ester from the pipet into a beaker of warm water, and cautiously waft the vapors toward your nose. Remember that the odor of an ester is very concentrated. Several sniffs may be necessary for you to identify the odor of the ester. Record which esters you prepared and their aromas. Part 2 : Destruction of Esters Esters may be destroyed by reversing the esterification reaction: water and an ester will react with one another (hydrolysis) to give an alcohol and an organic acid. This reaction is often carried out using sodium hydroxide as catalyst (in which case the sodium salt of the organic acid results, rather than the acid itself). R-COOH + HO-R' R-CO-OR' + H20 Set up a water bath in a 250-mL beaker on the hotplate. Adjust the heating control to maintain a temperature of approximately 70°C in the water bath. Obtain 10 drops of methyl salicylate in a clean test tube. Note the odor of the ester by cautiously wafting vapors from the test tube toward your nose. Add 2-3 mL of water to the test tube, followed by 10-15 drops of 20% NaOH solution (Caution!). Heat the methyl salicylate/NaOH mixture in the water bath for 15-20 minutes. After this heating period, again note the odor of the mixture. If the odor of mint is still noticeable, heat the test tube for an additional 10-minute period. Exp 10: The Preparation and Properties of Esters -3- Questions 1. Ordinarily, esterification reactions come to equilibrium before the full theoretical yield of ester is realized. Aside from the distillation discussed earlier, how might one experimentally shift the equilibrium of the esterification reaction so that a larger amount of ester might be isolated? 2. Using a Chemical Handbook, encyclopedia of Chemistry or Google, list two additional fragrant esters that were not discussed ion this experiment. 3. Draw the structure of each of the esters listed in the Table of Common Esters on the first page of this experiment. 4. Use another resource to write the definition for hydrolysis. Write the equation for the hydrolysis of methyl salicylate. 5. Esters are frequently used as additives in commercial products found in the home. Examine the labels of things you may have at home, such as shampoos, hand creams, and prepared foods, and find the names of two esters among the ingredients. List here the products and the esters you found.