* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Survey
Document related concepts
Transcript
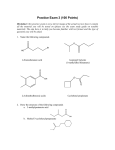
Kimberly Pambid 1st period AP Biology 10/30/09 Free Response: Carbon Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbon is the 15th most abundant elements in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. It is present in all known lifeforms, and in the human body carbon is the second most abundant element by mass after oxygen. This abundance, together with the unique diversity of organic compounds and their unusual polymer-forming ability at the temperatures commonly encountered on Earth, make this element the chemical basis of all known life. It has an affinity for bonding with other small atoms, including other carbon atoms, and is capable of forming multiple stable covalent bonds with such atoms. As a result, carbon is known to form almost ten million different compounds; the large majority of all chemical compounds. Carbon also has the highest melting and sublimation point of all elements. Although it forms an extraordinary variety of compounds, most forms of carbon are comparatively unreactive under normal conditions. Carbon is present in the atmosphere as carbon dioxide in 0,03% in volume. Several minerals, like limestone, dolomite, gypsum and marble, contain carbonates. All the plants and live animals are formed by complex organic compounds where carbon is combined with hydrogen, oxygen, nitrogen and other elements. The remains of live plants and animals form deposits: of petroleum, asphalt and bitumen. The natural gas deposits contain compounds formed by carbon and hydrogen. In combination with oxygen in carbon dioxide, carbon is found in the Earth's atmosphere and dissolved in all water bodies. Hydrocarbons contain carbon as well—coal "reserves". With smaller amounts of calcium, magnesium, and iron, carbon is a major component in very large masses of carbonate rock. Carbon occurs in all known organic life and is the basis of organic chemistry. When united with hydrogen, it forms various flammable compounds called hydrocarbons which are important to industry as refrigerants, lubricants, solvents, as chemical feedstock for the manufacture of plastics and petrochemicals and as fossil fuels. When combined with oxygen and hydrogen, carbon can form many groups of important biological compounds including sugars, lignans, chitins, alcohols, fats, and aromatic esters. With nitrogen it forms alkaloids, and with the addition of sulfur also it forms antibiotics, amino acids, and rubber products. With the addition of phosphorus to these other elements, it forms DNA and RNA, the chemical-code carriers of life, and adenosine triphosphate (ATP), the most important energy-transfer molecule in all living cells. The most prominent oxide is carbon dioxide. This was once the principal constituent of the paleoatmosphere, but is a minor component of the Earth's atmosphere today. Dissolved in water, it forms carbonic acid, but as most compounds with multiple single-bonded oxygens on a single carbon it is unstable. Through this intermediate, though, resonance-stabilized carbonate ions are produced. Some important minerals are carbonates, notably calcite. Carbon disulfide is similar. The other common oxide is carbon monoxide. It is formed by incomplete combustion, and is a colorless, odorless gas. The molecules each contain a triple bond and are fairly polar, resulting in a tendency to bind permanently to hemoglobin molecules, displacing oxygen, which has a lower binding affinity. Too much carbon dioxide in the atmosphere results in the greenhouse effect, which is the heating of the surface of a planet or moon due to the presence of an atmosphere containing gases that absorb and emit infrared radiation. Thus, greenhouse gases trap heat within the surface-troposphere system. This mechanism is fundamentally different from that of an actual greenhouse, which works by isolating warm air inside the structure so that heat is not lost by convection. Carbon dioxide is the human-produced greenhouse gas that contributes most of radioactive forcing from human activity. CO2 is produced by fossil fuel burning and other human activities such as cement production and tropical deforestation. The effect of combustion-produced carbon dioxide on the global climate, a special case of the greenhouse effect. A carboxyl group is a set of four atoms bonded together and present in carboxylic acids, including amino acids. Straight chain mono- and dicarboxylic acids occur in many natural settings and many are produced industrially on a large scale. They are used in the production of polymers, pharmaceuticals, solvent, and food additives. Vinegar, a dilute solution of acetic acid, is biologically produced from the fermentation of ethanol. It is used in food and beverages, but is not used in industry. Important carboxylic acids include acrylic and methacrylic acids which are used in polymer synthesis, adipic acid, citric acid, fatty acids, maleic acid (polymers), propionic acid (food preservative), terephthalic acid (polymers), and amino acids which are the building blocks of proteins that is important to the structure and function of living organisms. Carboxylic acids are also found in nutritional supplements used by humans. All fats consist of fatty acids bonded to a backbone structure, often glycerol. Chemically, this is a triester of glycerol, an ester being the molecule formed from the reaction of the carboxylic acid and an organic alcohol. Fats play a vital role in maintaining healthy skin and hair, insulating body organs against shock, maintaining body temperature, and promoting healthy cell function. Fats also serve as energy stores for the body, containing about. They are broken down in the body to release glycerol and free fatty acids. The glycerol can be converted to glucose by the liver and thus used as a source of energy. Fat also serves as a useful buffer towards a host of diseases. When a particular substance, whether chemical or biotic—reaches unsafe levels in the bloodstream, the body can effectively dilute—or at least maintain equilibrium of—the offending substances by storing it in new fat tissue. This helps to protect vital organs, until such time as the offending substances can be metabolized and/or removed from the body by such means as excretion, urination, accidental or intentional bloodletting, sebum excretion, and hair growth. While it is nearly impossible to remove fat completely from the diet, it would be unhealthy to do so. Some fatty acids are essential nutrients, meaning that they can't be produced in the body from other compounds and need to be consumed in small amounts. All other fats required by the body are non-essential and can be produced in the body from other compounds.