* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Aromatic Compounds
Ring-closing metathesis wikipedia , lookup
Marcus theory wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Homoaromaticity wikipedia , lookup
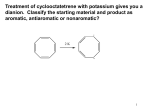
Aromatic Compounds • In 1825 Michael Faraday isolated a compound which boils at 80o and had a H:C ratio of 1:1 • It was later synthesized from PhCO2H isolated from gum benzoin, it was found to have a MW of 78 amu (C6H6) and hence called benzene. • Numerous compounds related to benzene with low C:H ratio and pleasant aroma has been discovered in the 19th century; hence called AROMATIC. • In 1866 Kekulé propose a cyclic structure with three double bonds. 1.397 Ao = Resonance representation 1.34 Ao 1.43 Ao Benzene show some unusual reactions OH KMnO4 / H2O + MnO2 OH cyclohexene KMnO4 / H2O No reaction Benzene Br2 / CCl4 Br Br cyclohexene Br Br2.FeBr3 Br2 / CCl4 Benzene No reaction Benzene + HBr Unusual stability of benzene Molar heat of hydration H2 catalyst ∆ Hobs = -120.1 KJmol-1 cyclohexene H2 catalyst ∆ Hobs = -232.7 KJmol-1 ∆ Hcal = -240.2 KJmol-1 1,3 cyclohexadiene H2 catalyst benzene ∆ Hobs = -209.2 KJmol-1 ∆ Hcal = -360.3 KJmol-1 151.4 KJmol-1 ∆Hobs is much less (151.4 KJmol-1) than predicted. That is benzene is more stable by 151.4 KJmol-1 than the hypothetical “cyclohexatriene” This energy difference is called RESONANCE ENERGY Proposed theories for the explanation of benzene unusual stability • Resonance or valence bond theory (VBT) • Molecular orbital theory (MOT) Valence Bond Theory When two or more structures for the same molecule can be drawn that differ only in the position of the electrons, then none of these structures will truly indicate the physical and chemical properties of that molecule. Resonance or Connonical forms “All aromatic compounds obey the V.B.T. However not all compounds that obey the V.B.T. are aromatic”. Failure of V.B.T Not all ANNULENES show similar stability to benzene [4] annulene [6] annulene [8] annulene Cyclooctatetraene~: Readily decolourize Br2 Oxidize by MnO4 Cyclobutadiene~: Very difficult to isolate pure. Molecular Orbital Theory Structure of benzene Six sp2 hybrid carbon atoms each with an unhybridized porbital that overlaps with the p-orbital of its neighbours to form a continuous ring of orbital above & below the plane of carbon atoms. The six π electrons are contained in this ring of overlapping orbitals. H H C C H C C H C H 120O H Apply the principles of MOT • σ bonds form the basic framework of the molecule. • The π electrons are placed in MO obeying the following rules. (i) There are as many MO as carbon atoms in the system. (ii) There is always an orbital of lowest energy which is single and can accommodate 2π electrons. (iii) The remaining orbitals occur in pairs which are degenerate and can accommodate 4π electrons. (iv) For even # of carbon atoms, the last pair of orbitals is followed by a single orbital of highest energy. • There is a certain number of π electron to impart stability. MO diagram of benzene E antibonding (node) nonbonding bonding NOTE (i) All bonding orbitals are full. (ii) No electron in anti-bonding orbitals (iii) All electrons have their spin paired. This configuration where the molecule having a closed bonding shell is energetically very favorable. Consider cyclobutadiene E antibonding (node) nonbonding bonding There are unpaired π electrons in non-bonding orbitals. Therefore do not have a closed bonding shell, hence not aromatic Requirements for aromaticity Compound must have a planar cyclic structure with each atom in the ring having an unhybridized p-orbital which form a continuous ring of parallel orbital in which the π electrons are delocalized resulting in a lowering of the electronic energy. Predicting aromaticity Hückel Rule If the number of π electrons in the cyclic system is 4N+2, where N is an integer, the system is aromatic. ie. N = 0, 1, 2, 3…… πe- = 2, 6, 10, 14……. Non-aromatic~: Does not have a continuous ring of porbitals. eg. Antiaromatic~: contain 4N π electrons ie 4, 8, 12…... O aromatic antiaromatic aromatic aromatic antiaromatic NOMENCLATURE Two systems are used in naming monosubstituted benzenes (i) Benzene is used as the parent name and the substituent is simply indicated by a prefix. NO2 Br Bromobenzene Nitrobenzene Cl Chlorobenzene (ii) The substituent & benzene ring taken together to form a new parent NH2 CH3 OH Methylbenzene Hydroxybenzene Aminobenzene Toluene Phenol Aniline O OCH3 C Methoxybenzene Anisole COOH SO3H CH3 Methyl phenyl ketone Benzoic acid Benzenesulfonic acid Acetophenone When two substituents are present, there relative positions are indicated by the prefixes ORTHO, META & PARA (abbreviated o-, m-, p- or by use of numbers) x ortho 1,2- meta 1,3- y para 1,4- Br I NO2 Br Cl o-dibromobenzene m-chloronitrobenzene CH3 p-iodotoluene When three or more substituents are on the benzene ring, numbers are used to give their positions. Assign the numbers exactely as you would with a substituted cyclohexane. The carbon atom bearing the functional group that defines the base name is assumed to be C-1. Br CH3 OH NO2 Br NO2 Br 1,3,5-tribromobenzene NO2 2,4-dinitrotoluene I 4-iodo-2-nitrophenol REACTIONS OF BENZENE Benzene is an electron rich molecule and hence is a center of high electron density, it therefore undergo electrophilic aromatic substitution (E. A. S) E H + E + electrophile H Mechanism of E. A. S. (i) + E R. D. S. slow H H E E H E (ii) Rearomatisation H E fast E + H Reaction Energy Profile δ+ PE H E TS 1 δ+ E TS2 H + H E σ-complex + E EAct1 EAct2 E + Reaction Progress H Some reactions of benzene Nitration NO2 + H2SO4 HNO3 + H2O (i) Generation of the electrophile; nitronium ion (a) H2SO4 (b) H2O (c) H2SO4 + H2O 2 H2SO4 + HNO3 OVERALL + HO NO2 NO2 H2O NO2 + H2O + NO2 + HSO4 + H3O H3O NO2 O HSO4 + 2 HSO4 N O (ii) Addition of electrophile (+NO2) H O R. D. S. N NO2 O (iii) Rearomatization The σ-complex, once formed will only react in a forward direction since the activation energy for the expulsion of +NO2 is much higher than for loss of the proton. H NO2 HSO4 fast NO2 + H2SO4 Sulfonation of benzene SO3H fuming H2SO4 + H2O O (i) Generation of the electrophile; sulfur trioxide O SO3 2 H2SO4 + H3O + HSO4 (ii) Addition of electrophile (SO3) H O S O O R. D. S. O S O O S O (iii) Rearomatization H O O S HSO4 O S fast O O O O S O O H O SO3H SO3H + HSO4 Halogenation of benzene E.A.S. on benzene by halides requires the assistance of Lewis acid catalyst. X + X2 FeX3 + HX X = Cl or Br e.g. bromonation of benzene (i) Generation of the electrophile Br Br + FeBr3 Br Br FeBr3 Br BrFeBr3 (ii) Addition of electrophile H + Br Br Br FeBr3 + FeBr4 (iii) Rearomatization H Br FeBr3 Br fast Br + HBr + FeBr3 Alkylation of benzene The Friedel-Crafts Reaction Chloro and bromoalkanes react with benzene in the presence of Lewis acid catalysts to give alkylbenzenes R + RX AlX3 HX + X = Cl or Br e.g. Ethylation of benzene CH2CH3 + CH3CH2Cl AlCl3 + HCl (i) Generation of the electrophile CH3CH2 Cl AlCl3 + CH3CH2 Cl AlCl3 CH3CH2 AlCl4 (ii) Addition of electrophile H + CH2CH3 Cl CH2CH3 AlCl3 + AlCl4 (iii) Rearomatization H CH2CH3 Cl AlCl3 fast CH2CH3 + HCl + AlCl3 Alkylation with other reagents The electrophile is essentially a carbocation (R+). Compounds capable of generating such species may be used in the Friedel-Crafts alkylation. e.g. alcohols alkenes OH H OH2 -H2O H Once electrophile is formed, then proceed as in previous slide. Limitations of Friedel-Craft rxn. (i) When R+ formed from an from alkylhalides, alkenes or alcohols, can rearrange to a more stable R+ it usually does so. e.g. CH3 CH CH2OH CH3 CH CH2 CH3 CH3 CH3 C CH3 (ii) Reaction is difficult to stop at monoalkylation, i.e. R polyalkylation often occur. R R R e.g. RX Lewis acid + + R (i) Aryl and vinylic halides cannot be used as the halide component because they do not form R+ readily. e.g. RCH CHX RCH CH CH3 O Friedel-Craft acylation of benzene R C Similar to the Friedel-Craft alkylation except that the haloalkane is replaced by an acyl halide to produce the corresponding ketone. O C + AlCl3 RCOCl R + (i) Generation of the electrophile O R C O Cl + AlCl3 R C R O C Cl R C acylium ion AlCl3 O + AlCl4 HCl (ii) Addition of electrophile R H C C O R O (iii) Rearomatization O H C O C R Cl AlCl3 R + HCl + AlCl3 Advantages of acylation vs. alkylation (i) Rearrangement of the acyl group does not occur. (ii) Acylation produce only monosubstituted product. (iii) Easily reduced products. O C R CH2R alkylbenzene