* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter Review- Josh and Niels 1. Rutherford`s Atom • Rutherford`s
Survey
Document related concepts
Transcript
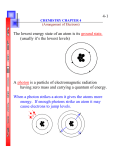
Chapter Review- Josh and Niels 1. Rutherford’s Atom Rutherford’s experiment Shot alpha particles at gold foil and they bounced in different directions Shows that there we different things in the atom Didn’t show what electrons were doing 2. Energy and Light: Electromagnetic Radiation: Energy transmitted from one placet o another Many kinds of light o Gamma o X Ray o Ultraviolet o Visible o Infrared o Microwaves o Radio-waves Wavelength: Distance between two consecutive wave peaks Frequency: Indicates how many wave peaks pass a certain point per given time period Photons: Stream of tiny packets of energy 3. Emission of Energy by Atoms Ions release different colors o From Atoms release energy Atom becomes excited an goes to lower energy stage and goes down the steps 4. The Energy Levels of Hydrogen The change in energy when it goes from excited state to lower state creates a color Quantized: Only certain values are allowed Only certain values happen in the case of energy emission Atom only has certain amount of atom 5. The Bohr Model of the Atom Bohr used hydrogen to construct a model of the atom Came up with a dot in middle (nucleus) and circles around it for the atoms to go into o Wrong because different shapes per some orbitals such as p,d,f 6. The Wave Mechanical Model of the Atom Wave Mechanical Model: Bohr’s model except without two dimensional, Circular orbitals. (It contains the s, p, d, etc. orbitals), also makes electrons waves The wave mechanical model explains atoms by postulating that the electron has both wave and particle characteristics. 7. The Hydrogen Orbitals Orbital: The location and movement of electrons Principal energy levels: Electrons are divided into levels depending on their energy level. Sublevels- The further division of energy levels within each principal energy level. The number of sublevels increases as the principal energy level increases. 8. The Wave Mechanical Model (further development) Pauli Exclusion principle: States that an atomic orbital can hold a maximum of two electrons, and those two electrons must have opposite spins. Principal Components of the Wave Mechanical Model of the atom o 1. Atoms have a series of energy levels called principal energy levels, which are designated by whole numbers symbolized by n; n can equal 1,2,3,4... Level 1 4d 5s 4p 3d 4s 3p 3s 2p corresponds to n=1, level 2 corresponds to n=2, and so on. o 2. The energy of the level increases as the value of n increases. o 3. Each principal energy level contains one or more types of orbitals, called sublevels. o 4. The number of sublevels present in a given principal energy level equals n. For example, level 1 contains one sublevel (1s); level 2 contains two sublevels (two types of orbital’s), the 2s orbital and the three 2p orbital’s; and so on. o 5. The n value is always used to label the orbitals of a given principal level and is followed by the letter that indicates the type of the orbital. For example, the designation 3p means an orbital in level 3 that has two lobes. o 6. An orbital can be empty or it can contain one or two electrons, but never more than two. They must have opposite spins. o 7. The shape only indicates the probability distribution. 9. Electron Arrangements in the First Eighteen Atoms on the Periodic Table Electron Configuration: An electron arrangement Orbital Diagram: Another way of showing electron configuration and is also known as a box diagram. Valence Electrons: The electrons in the outermost (highest) principal energy level of an atom. Core Electrons: The inner electrons, which are not involved in bonding atoms to each other. 10. Electron Configurations and the Periodic Table Main Group Elements: The elements in groups 1,2,3,4,5,6,7, and 8. These elements are also called representative elements 11. Atomic Properties and the Periodic Table Metals: Typically have the following physical properties, a lustrous appearance, the ability to change shape without breaking, and being good conductors of heat and electricity. Nonmetals: The opposite of a metal, are bad conductors of heat and electricity, don’t have a lustrous appearance etc. Metalloids- Elements that exhibit both metallic and nonmetallic behavior. Atomic Size: Atoms get larger as we go down a group on the periodic table and get smaller as we go from left to right across the period. Chapter 11 Review Part 1: Vocab 1. What are the two ways to represent electron configuration? 2. What are the electrons on the outmost part of the shell called? 3. What are electrons that are on the inside of the electron configuration called? 4. On the periodic table there are two rows that are separate from the main table, they both have special names, what is the name of the top one? 5. What is the name for elements in groups 1-8 Metals? 6. Describe the features of a metal and where they are on the periodic table? 7. Describe the features of a non-metal and where they are on the periodic table? 8. Describe the features of a metaloid and where they are on the periodic table Atomic size 9. What is the energy required to remove an electron from an individual atom in the gas phase called? 10. The heat you feel from the sun, or the heat you feel when you put your hands next to a fire is an example of what? 11. What is symbolized by the Greek letter lambda, and represents the distance between two wave peaks? 12. What indicates how many wave peaks pass a certain point per given period? 13. What is a stream of tiny packets of energy called? 14. What is it called when only certain values are allowed? For example, we say that the energy levels of hydrogen are (blank) 15. What was Schrodinger’s model of the atom called? 16. What is the area where it is possible to locate electrons called? 17. What are the different energy levels called? Or what do we use to represent how a hydrogen atom is organized? 18. The principle energy levels are divided further into smaller groups, what are these groups called? 19. What states that an atomic orbital can hold a maximum of two electrons and those two electrons must have opposite spins. 20. What do we use to show the way electrons are arranged in an element? Part 2: 1. Describe Rutherford’s model of the atom. and explain how he came up with it? 2. Why did Bohr's model of the atom not work well for other elements? 3. What is the electron configuration for Iodine (53)? 4. What do the S and P orbitals look like? Draw a diagram. 5. Do a box form for Lithium? 6. Give two examples of electromagnetic radiation? 7. How does the wavelength of atomic frequency differ from its frequency? 8. Which element has the electron configuration of, 1s2 2s2 2p3? 9. Why was Bohr’s theory for the hydrogen atom initially accepted, and why was it later not accepted? 10. What is the Pauli exclusion principle, and how many electrons can occupy an orbital? Part 3: 1. Calculate the number of moles in 160 grams of the molecule with the electron configuration of 1s2, 2s2 3p4 2. Is releasing energy from an atom in the form of a photon an exothermic or endothermic reaction? 3. Bond the following elements and name them in the answer (neutral charge for electron configuration): 1s2, 2s2, 2p6, 3s2, 3p4 AND 1s2, 2s2, 2p4 4. Write the electron configuration for an ion with atomic number 16, and has a charge of -2 5. An atom with ground state electronic configuration of 1s2 2s2 2p5 is most likely to form a charge of? Part 1 Answers 1. 2. 3. 4. 5. 6. Orbital diagram and box diagram Valence electrons Core Electrons Lanthanide series Main-group elements A metal is conductive, lustrous appearance, the ability to change shape without breaking. Metals are found to the left of the black line. 7. A non-metal isn’t conductive, doesn’t have a lustrous appearance and doesn’t usually change shape without breaking. They are found on the periodic table to the right of the line, but don’t' touch it 8. Exhibit both metal and non-metal behaviors. Found on the dark line of your periodic table. 9. Ionization energy 10. Electromagnetic radiation 11. Wavelength 12. Frequency 13. Photon 14. Quantized Energy 15. Wave mechanical model 16. Orbital 17. Principle energy levels 18. Sublevels 19. Pauli exclusion principle 20. Electronic Configuration Part 2 Answers: 1. 1s2 2s2 2p6 3s2 3p4 2. The Bohr model of the atom was that you had a nucleus and orbitals that were circular. It doesn’t work well for most elements because the shapes are not all circles. 3. 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 4d10, 4p6, 5s2, 5d10, 5p5 4. 5. “<” = up arrow and vice versa [<>] [<] 6. X-rays, and radio waves that transmit music and voices. 7. The wavelength is the distance between two consecutive wave peaks, and the frequency indicates how many wave peaks pass a certain point per given period. 8. Nitrogen 9. Bohr’s theory explained the line spectrum of hydrogen exactly but did not explain measurements for other atoms other than hydrogen. 10. It states that an orbital can hold a maximum of to electrons, and those two electrons must have an opposite spin. Part 3 answer key 1. It is oxygen, which has an atomic mass of 16 so you divide 160 by 16 and get 16 moles. 2. Exothermic 3. The elements are Sulfur and Oxygen and they would combine with a double bond. 4. 1s2 2s2 2p6 3s2 3p6 5. -1