* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download C. - Knights of The Periodic Table
Process chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Rigid rotor wikipedia , lookup
Marcus theory wikipedia , lookup
Chemical bond wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Chemical reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Host–guest chemistry wikipedia , lookup
Atomic theory wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Molecular dynamics wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Click chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Transition state theory wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
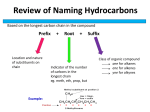
Nomenclature Chemical Formulas Reactions Which is an example of a synthesis reaction? A. HCl + KOH KCl + H2O B. Pb(NO3)2 + 2HBr PbBr2 + 2HNO3 C. C + O2 CO2 D. Mg + H2SO4 MgSO4 + H2 A + B AB 2 A. 1, 1, 1 B. 1, 1, 2 C. 2, 1, 2 D. 2, 2, 1 2 The coefficients of the correctly balanced equation for the reaction illustrated are — Which of the following equations is balanced? What would be the product(s) of this reaction? 3MgO + 2Al 3 Mg + Al2O3 A. 2Mg3Al2O2 B. Mg3Al2 + 3O2 C. 6Mg + Al3O2 D. 3Mg + Al2O3 Elements from which two groups in the periodic table would most likely combine with each other to form an ionic compound? A. 1 and 2 B. 16 and 17 C. 1 and 17 D. 17 and 18 The diagram is a potential energy curve for a reaction. Which number represents the effect of a catalyst on the reaction? A. 1 B. 2 C. 3 D. 4 Which is the correct formula for iron (III) sulfate? A. Fe3(SO4)2 B. FeSO4 C. Fe2(SO4)3 D. Fe2(SO3)3 Remember the Roman Numeral is the charge of the Iron *Fe’s oxidation number Fe3+ SO42- Which of these represents the empirical formula and the molecular formula, respectively, for a given organic compound? A. CH and C2H2 B. CH and CH4 C. CH2 and C2H2 D. CH3 and C3H12 Empirical formula is the simplest whole number ratio Molecular formula is a multiple of the Empirical formula The system that shows a decrease in entropy (disorder) is — A. B. C. D. air escaping from a tire snow melting salt dissolving in water water freezing In chemical compounds, covalent bonds form when — A. the electronegativity difference between two atoms is very large B. electrons are completely transferred between two metals C. pairs of electrons are shared between two nonmetal atoms D. two nonmetal ions are attracted to each other by opposite charges Covalent bonds result from sharing electrons Each beaker shown above contains 2.2 grams of iron and 1 liter of 3M H2SO4 at STP. Which reaction will go to completion first and More surface area! why? A. Beaker A because of increased surface area B. Beaker B because of increased surface area C. Beaker A because of a higher concentration level D. Beaker B because of a higher concentration level A catalyst is a substance used in chemical reactions to — A. provide a higher activation energy pathway B. decrease collisions between reactant molecules C. increase the rate of the reaction D. change the equilibrium to favor products Catalysts speeds up a chemical reaction by lowering the activation energy! Which reaction type best describes the reaction above? A. Combination B. Decomposition C. Single replacement D. Combustion 1 3 ?3 AgNO3 + ?AlCl 1 3 → ?AgCl + ?Al(NO 3)3 Which of these sets of coefficients will balance this equation? A. 3, 3, 2, 1 B. 3, 1, 3, 1 C. 1, 6, 1, 9 D. 9, 3, 3, 3 The formula for lithium nitride is — A. LiN B. Li3N C. Li3N3 D. NLi3 Li+ N3- This diagram of a chemical reaction shows that the reaction is — More energy in products energy went into products A. endothermic B. exothermic C. reversible D. at equilibrium Ni(C2H3O2)4 How many atoms are represented in this formula? 1 + 4(2) + 4(3) + 4(2) = 29 A. 5 B. 8 C. 28 D. 29 The correct structural formula for C2H4 is Carbon forms 4 bonds! Only 2 hydrogen What type of reaction does this illustration represent? AB A. Decomposition B. Synthesis C. Single-replacement D. Double-replacement A+B Which of these reactions shows simple chemical decomposition? A compound has 50% sulfur and 50% oxygen. What is its empirical formula? Percent to mass Mass to mole Divide by small Multiply to whole A compound has 50% sulfur and 50% oxygen. What is its empirical formula? 50 gS 1molS x 1.56molS / 1.56 1 32.1gS 50 gO 1molO x 3.13molO / 1.56 2 16 gO A compound has 50% sulfur and 50% oxygen. What is its empirical formula? A. B. C. D. SO4 S2O4 SO3 SO2 The Lewis electron dot system represents electrons in the — A. outer energy level B. inner level C. middle level D. core level A(s) + B(s) D(g) + heat The reaction shown above is — Heat is a product therefore the reaction is … A. an endothermic reaction B. an exothermic reaction C. a decomposition reaction D. a double-replacement reaction The correct name for MgI2 is — A. magnesium iodide B. magnesium iodite C. magnesium (II) iodide D. magnesium diiodide Mg2+ Magnesium Ion I2 actually 2 I- Iodide ion If the diagram were the correct representation for the Lewis structure of a molecule, then the X would be representative of the element — A. oxygen B. fluorine C. nitrogen D. sulfur Exception Chlorine and bromine are in the same family in the periodic table. According to the information in the table, what would be the correct formula for sodium bromate? A. NaBrO B. Na2BrO C. Na3BrO3 D. NaBrO3 Which of the groups below has the electron dot structure shown above? 7 dots = 7 valance electrons which is group 17 A . Noble gases B. Halogens C. Alkali metals D. Transition elements When naming a transition metal that has more than one oxidation number, the numeric value of the oxidation number is indicated by a — A. Roman numeral B. Greek prefix C. subscript D. suffix A compound is composed of 85.64% carbon and 14.36% hydrogen. The compound has a formula mass of 42.08 grams. What is the molecular formula? A. CH2 B. C3H6 C. C2H4 D. C2H18 Change % to mass to moles divide by small 85.64 gC 1 mol C = 7.137 / 7.137 = 1 C 12 g C 14.36 g H 1 mol H = 14.36 / 7.137 = 2 H 1gH Calculate the mass of the empirical formula CH2 1(12) + 2(1) = 14 Divide into molecular mass 42.08/14 = 3 Multiply by the empirical formula 3(CH2) = C3H6 Another way…. A. CH2 1(12) + 2(1) = 14 g B. C3H6 3(12) + 6(1) = 42 g C. C2H4 2(12) + 4(1) = 28 g D. C2H18 2(12) + 18(1) = 42 g The molar mass was 42 and there are two answers that are 42 but C2H18 is a formula that is not possible carbon only forms 4 bonds! Therefore B is the correct answer. A compound is composed of 85.64% carbon and 14.36% hydrogen. The compound has a formula mass of 42.08 grams. What is the molecular formula? A. CH2 B. C3H6 C. C2H4 D. C2H18 Which of the following is the correct molecular shape of CH4? A. Bent B. Linear C. Pyramidal D. Tetrahedral According to the table, which of these probably has the strongest bonds? Highest BP and MP! A. Hydrogen gas B. Iron crystals C. Sodium chloride D. Water The empirical formula for ethyne (C2H2) is — Empirical formula = simplest formula A. CH B. C2H2 C. CH2 D. C2H The type of reaction represented by the above equation is — A. single-replacement B. double-replacement C. synthesis D. decomposition 2 3 2 3 When this equation is correctly balanced, the coefficient of the AlCl3 will be — A. 1 B. 2 C. 4 D. 6 A catalyst accelerates a chemical reaction because the — A. catalyst decreases the number of collisions in a reaction B. activation energy of the reaction is lowered in the presence of a catalyst C. catalyst decreases the concentration of the reactants D. temperature of the reaction increases due to the catalyst A compound is composed of 58.8% C, 9.8% H, and 31.4% O, and the molar mass is 102 g/mol. What is the molecular formula for this compound? A. C2H10O 3 B. C5H5O3 C. C5H10O2 D. CH3O3 Find Empirical Formula Change % to mass Mass to Mole Dividie by small Multiply to whole Find multiple of empirical formula to molecular formula by Mass Or you can find the molar mass of each formula in answer choices and see which one equals 102…. C. C5H10O2 5(12) + 10(1) + 2(160) = 102 g A compound is composed of 58.8% C, 9.8% H, and 31.4% O, and the molar mass is 102 g/mol. What is the molecular formula for this compound? A. C2H10O 3 B. C5H5O3 C. C5H10O2 D. CH3O3 Which of these completes this reaction? A. Oxygen B. Hydrogen C. Metal oxide D. Air Example: 2Na + 2HOH 2NaOH + H2 A balanced chemical equation has equal numbers of atoms of each type on both sides of the equation. This illustrates the principle of — A. conservation of energy B. conservation of mass C. action and reaction D. natural selection The type of bond found in magnesium chloride is — Magnesium group 2 (metal) Chlorine group 17 (nonmetal) A. covalent B. nonpolar C. ionic D. metallic If the temperature of a reaction is increased, the reaction proceeds at a much quicker rate because the — A. activation energy increases B. energy of the products increases C. frequency of collisions between reactants increases D. energy of the activated complex increases In the above reaction, a cloudiness at completion due to colloidal suspension of sulfur appears. If the reaction is carried out at various temperatures, at which temperature would it proceed at the fastest rate? A. 20º C B. 30º C C. 40º C Higher Temperature faster rate! D. 50º C The formula H2SO4 is representative of which of the following? A. A catalyst B. A base C. An acid D. An organic compound A heated liquid placed in a closed container will vaporize until — A. the boundary between liquid and vapor disappears B. all the liquid molecules become vapor molecules C. the number of liquid molecules vaporizing equals the number of vapor molecules condensing D. the vapor pressure is greater than the atmospheric pressure Which compound contains both ionic and covalent bonds? A. NH4Cl B. MgBr2 C. CH4 D. NH3 Polyatomic ions are held together by covalent bonds! Which of the following containers of water best shows dynamic equilibrium between vapor and liquid? Open jar To indicate the number of atoms of each element present in a molecular compound, scientists use — A. Roman numerals B. superscripts C. prefixes D. subscripts CH4 Using the chart, which of these combinations will probably form a precipitate? A. Ammonium chloride B. Barium bromide C. Calcium chromate D. Copper (II) carbonate The correct name for the compound CCl4 is — A. carbon tetrachloride B. carbon chloride C. monocarbon chloride D. tetracarbon monochloride Which of these is the general formula for a double-replacement reaction? A. B. C. D. A +B AB AB + XY BA + YX AB + XY AY + XB A + B + XY AX + BY The correct formula of an ionic compound containing Al3+ and CO32- is — Al3+ CO 23 A. B. C. D. AlCO3 Al(CO3)3 Al2(CO3)3 Al3(CO3)2 Aluminum +Sulfuric Acid Aluminum Sulfate + Hydrogen Gas Which of the following is the balanced chemical equation for the reaction shown above? Which answer is balanced????? A. Al + H2SO4 Al2(SO4)3 + H2 B. 2Al + 3H2SO4 Al2(SO4)3 + 3H2 C. 2Al + 3H2SO4 Al2(SO4)3 + H2 D. 2Al + H2SO4 Al2(SO4)3 + H2 The figure shows a compound containing hydrogen (H) and an unknown element Z. To which group on the periodic table does element Z belong? A. 13 B. 14 C. 15 D. 16 The element Z forms three bonds which means it has 3 valance electrons in addition to the pair show. That gives it 5 valance electrons. Which group has 5 valance electrons. Which of the following is the name of the molecule PCl3? A. Phosphorus trichloride B. Phosphorus chloride C. Potassium trichloride D. Potassium chloride The appropriate model for a decomposition reaction is — The formula for dinitrogen tetroxide is — A. N2O4 B. N3O3 C. N2O2 D. NO dinitrogen tetroxide What is the correct Lewis dot structure for arsenic? Arsenic (As) is in group 15! Which means 5 valance electrons Or 5 dots! The empirical formula for a substance is CH2. If the molecular mass of the substance is 56, the molecular formula is A. C2H4 B. C3H6 C. C4H8 D. C5H10 Molar Mass CH2 = 1(12) + 2(12) = 14 56/14 = 4 4(CH2) = H2O(l) H2O(g) Water molecules in a sealed jar are in a state of dynamic equilibrium because water vapor molecules — Equilibrium same rate condensing and evaporating A. are condensing at the same rate that others are evaporating B. cease to form when the air in the jar becomes saturated C. are evaporating faster than they are condensing D. form only at high temperatures The diagram shows the structural formula of benzene. The empirical and the molecular formulas of benzene are, respectively — A. CH, C2H2 B. CH, C3H3 C. C3H3, C6H6 D. CH, C6H6 6 Carbon atoms and 6 Hydrogen atoms Divide by 6 gives you ………. 2Al AlCl 2AlCl Al ++ 6HCl HCl 3 + H3H 3+ 2 2 When the above equation is balanced, the coefficient of the hydrochloric acid will be A. 2 B. 3 C. 4 D. 6 2NO(g) + O2(g) 2NO2(g) ΔH = - 27 kcal Which graph represents the reaction shown above? ΔH = - 27 kcal Means the reaction was exothermic releasing energy. Therefore the products have less energy then the reactants 2NO(g) + O2(g) 2NO2(g) ΔH = - 27 kcal Which graph represents the reaction shown above? Which Graph shows less energy in products than reactants? ? ? ? ? ? ? ? Which of the following occurs when a reaction in a solution is at equilibrium and more product is added to the solution? A. Equilibrium shifts to produce more product B. Equilibrium shifts to produce more reactant C. No change will occur D. The reaction will stop What shape does the molecule BF3 have? A. Bent B. Linear C. Tetrahedral D. Trigonal planar Boron (B) is in group 13 therefore B has three valance electrons. Fluorine (F) is a Halogen and therefore has 7 valance electrons F and B Bond by 3 bonds F B F F CaSO4 + Al(OH)3 Ca(OH)2 + Al2(SO4)3 3CaSO4 + 2Al(OH)3 3Ca(OH)2 + Al2(SO4)3 When the above equation is balanced, the coefficients in order are — A. 1, 1, 1, 1 B. 2, 1, 1, 2 C. 3, 1, 3, 2 D. 3, 2, 3, 1 The correct name for P2O5 is — A. phosphorus (V) pentoxide B. phosphorus oxide C. phosphorus (II) oxide D. diphosphorus pentoxide Which set of coefficients will balance this equation? A. 1, 3, 1, 1 B. 2, 3, 2, 6 C. 2, 6, 2, 3 D. 3, 6, 3, 2 Al + HCl AlCl3 + H2 2Al + 6HCl 2AlCl3 + 3H2 Using the table, what is the correct formula for ammonium phosphate? A. NH4PO4 B. (NH4)2(PO4)3 C. (NH4)3PO4 D. NH4(PO4)3 Which half-reaction represents reduction? A. Cu0 → Cu+2 + 2eB. Fe+2 → Fe+3 + 1eC. Ag+1 + 1e- → Ag0 D. Al0 → Al+3 + 3eRemember “LEO the lion goes GER” LEO Lose Electrons Oxidized GER Gain Electrons Reduced Which of the following best represents the reaction between hydrochloric acid and sodium hydroxide? A. 2HCl + 2NaOH → Na(OH)2 + H2Cl2 B. HCl2 + 2Na(OH)2 → 2H2O +2NaCl + OH C. HCl + NaOH → H2O + NaCl D. 2HCl + Na(OH)2 → 2H2 + NaCl + O2 The type of formula that shows the arrangements of atoms and bonds is called — Structural = Arrangement A. empirical B. chemical C. molecular D. structural What is the molecular formula of a substance that has an empirical formula of C2H5 and a molecular mass of 58 g/mole? A. C2H5 B. C5H2 C. C4H10 D. C6H15 Molar Mass C2H5 = 2(12) + 5(1) = 29g/mole 58/29 = 2 2(C2H5) = C4H10 Na2CO3 + Ca(OH)2 2NaOH + CaCO3 Which type of reaction is represented here? A. Single replacement B. Double replacement C. Synthesis D. Decomposition The empirical formula for C6H12 is — A. C3H6 B. C2H4 C. CH3 D. CH2 2O3 (g) 3 ______ (g) Which completes the chemical equation above? A. O2 B. O3 C. ClO D. ClO2 Total of 6 Oxygen An alien astronaut landed on Earth and created the periodic table shown. The astronaut was trying to determine what type of bond would be present in several compounds. The type of bond in a compound containing G and E would be — The type of bond in a compound containing G and E would be — A. a metallic bond B. a nonmetallic bond C. a covalent bond D. an ionic bond Calcium carbonate was placed in a flask on a balance, and dilute hydrochloric acid was added. Carbon dioxide that was produced escaped from the flask. The total mass of the flask and its contents was recorded every 10 seconds. The diagram above shows a plot of the results. Between which of the following times was the reaction the fastest? Between which of the following times was the reaction the fastest? A. 0 and 10 seconds B. 10 and 20 seconds C. 20 and 30 seconds D. 30 and 40 seconds Bonding between two elements of equal electronegativity would be — A. 100% covalent B. primarily ionic C. 50% ionic D. metallic in character The molar mass (gram formula mass) for the compound sodium thiosulfate, Na2S2O3, is— 2(23) + 2(32.1) + 3(16) = 158.2 A. 71 grams B. 153 grams C. 158 grams D. 254 grams The correct formula for copper (I) bromide is — A. CuBr B. CuBr2 C. Cu2Br D. Cu2Br3 Cu+ Br- Which of the following models a synthesis reaction?