* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch. 8 Notes (Chemical Reactions) Teacher Relearn
Chemical bond wikipedia , lookup
Chemical industry wikipedia , lookup
Asymmetric induction wikipedia , lookup
Water splitting wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Marcus theory wikipedia , lookup
Electrolysis of water wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
History of molecular theory wikipedia , lookup
Isotopic labeling wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Process chemistry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Nuclear chemistry wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Acid–base reaction wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Drug discovery wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
History of chemistry wikipedia , lookup
Organic chemistry wikipedia , lookup
Atomic theory wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Electrochemistry wikipedia , lookup
Rate equation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Click chemistry wikipedia , lookup
Transition state theory wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Chemical reaction wikipedia , lookup
Stoichiometry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
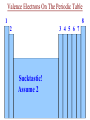
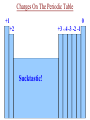
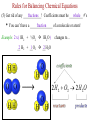
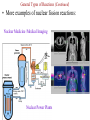
Ch. 6 Notes---Chemical Names & Formulas • Ionic Compounds (“________”): salts metal – Name or formula starts with a _________ (or NH4 +, ammonium). – Other quick ways to tell if the compound is ionic: • formula uses parentheses Ca(OH)2 Example: ________________ • formula contains more than 2 elements (capital letters) FeCrO4 Example: ________________ • name uses Roman numerals lead(II) chloride Example: ________________ • name ends in “-ate” or “ite”. barium sulfate Example: _________________ Valence Electrons On The Periodic Table 1 8 2 3 4 5 6 7 Sucktastic! Assume 2 Charges On The Periodic Table +1 0 +2 +3 +/-4 -3 -2 -1 Sucktastic! Writing Formulas for Ionic Compounds • • • Step 1-- use your ion sheet and find the ions and their charges. Step 2-- “Cross the charges” if they don’t balance out. Step 3-- Use parentheses around polyatomic ion “chunks”. Practice Problems: Write the formula for each ionic compound. copper(II) bromide +2 Br -1 Cu = CuBr2 …(don’t show 1’s) ____________________ aluminum nitrite +3 NO -1 Al = Al(NO2)3 2 _________________________ barium hydrogen carbonate Ba+2 HCO3-1 = Ba(HCO3)2 ___________________________ Naming Ionic Compounds • Just use your ion sheet and find the names of the ions. cation name anion name Practice Problems: Name the following ionic compounds. a) NaC2H3O2 b) (NH4)2CO3 c) Fe(OH)3 d) PbSO4 molecules Molecular Compounds (“____________”): nonmetal (exception: NH4 +) – Name or formula starts with a ____________ – Other quick ways to tell if the compound is molecular: • Name has prefixes and also ends in “-ide”. (It must have both!) carbon dioxide dinitrogen pentoxide Examples: _________________,_______________________ Naming Molecular Compounds • • charges You do not use the ion sheet for molecules because no __________ share are needed. They ______________ electrons instead of transferring them. prefixes Use ________________ to indicate the # and kind of atom in the compound. mono=1 di=2 tri=3 tetra=4 penta=5 hexa=6 hepta=7 octa=8 non=9 deca=10 • Use the general format shown below… prefix-(except mono)-name the 1st element prefix-name the 2nd element ending with -ide Practice Problems: Name the following molecules. N2O5 dinitrogen pentoxide CO carbon monoxide Cl4F7 tetrachlorine heptafluoride SO3 sulfur trioxide • Ch. 8 Notes -- Chemical Reactions Chemical equations give information in two major areas: Reactants products 1. _____________ and ______________ of the reaction. amount 2. Coefficients of a balanced chemical equation tell us the ______ of the substances involved. Example of a Balanced Chemical Equation: 2H2 (g) + O2 (g) 2H2O (g) left side of the arrow, and the Reactants are on the ______ right yields products are on the __________ side. The arrow means “________”, or “reacts to produce” when read aloud. • • From our example, hydrogen reacts with oxygen in a ___:___ 2 1 ratio. moles or The coefficients represent either the number of _________ molecules present. Common Symbols used in Chemical Equations + → = used to separate 2 reactants or 2 products from each other = “yields” or “reacts to produce” = _____________ reaction (like a rechargeable battery) reversible (s) (l) (g) (aq) = phase of matter: (solid, liquid, gas, or “aqueous”) heat = ___________ supplied to the reaction MnO2 = a catalyst, (in this case, MnO2), is used to ________ speed ____ up the reaction. Decoding Common Chemical Equation Symbols Practice Problems: Describe the following reactions using complete sentences. a) NaHCO3 (s) + HCl (aq) NaCl (aq) + H2O (l) + CO2 (g) Solid sodium bicarbonate plus aqueous hydrochloric acid yields aqueous sodium chloride plus liquid water plus carbon dioxide gas. b) H2SO4 (aq) + BaCl2 (aq) HCl (aq) + BaSO4 (s) Aqueous sulfuric acid plus aqueous barium chloride yields aqueous hydrochloric acid plus solid barium sulfate. c) Write a chemical equation from the following description: “Sodium plus bromine, when heated, reacts to produce solid sodium bromide.” Na(s) + Br2 (l) NaBr (s) Balancing Chemical Equations Why do you have to balance a chemical equation? • Law of Conservation of Matter (or Mass): “Matter is neither ____________ nor _______________ in chemical reactions.” created destroyed joined • During a chemical reaction, atoms are either _________, ______________, or rearranged. The _____________ and type of separated number each atom stays the same. How do you balance a chemical equation? • __________________ are placed in front of the substances involved Coefficients in the chemical reaction to get the same number of atoms of each element on both sides of the equation. This number will multiply the number of atoms there are in a formula. Rules for Balancing Chemical Equations (1) Coefficients can only be placed ___ in _________ front of a chemical formula. Practice Problems: How many atoms of each type are indicated in the following compounds? (a) 2 (NH4)3PO4 N= 6___ 24 P= ___ 2 O= ___ 8 H= ___ (b) 4 KC2H3O2 4 C= ___ 8 H= ___ 12 O= ___ 8 K= ___ (c) 3 Ca(NO3)2 3 N= ___ 18 6 O= ___ Ca= ___ Rules for Balancing Chemical Equations (2) You cannot change a ________________!! subscript Example : 2 H2 + O2 2 H2O H2 O2 To balance oxygen, you cannot change water’s formula to_________! (3) You cannot place the coefficient in the ______________ of a middle formula!! Example : 2Al + N2 2 AlN To balance nitrogen, you cannot put a 2 in the middle to make _______. Al2N whole # ratio. (4) Reduce the coefficients to the simplest ____________ ___ Example: 4H2 + 2O2 4H2O can be reduced to… __H 2 2 + __O 1 2 __H 2 2O Rules for Balancing Chemical Equations (5) Get rid of any ____________! Coefficients must be _________ fractions whole #’s • You can’t have a _______________ of a molecule or atom! fraction Example: 2 x ( 1H2 + ½O2 1H2O ) __ 2 H2 + __O 1 2 __H 2 2O changes to… Balancing Equations: “Helpful Hints” a) Balance elements that appear in more than one compound ________. last 1 ___(NH 4)2CO3 2 ___NH 3 + 1 ___CO 2 + 1 2O ___H ion “___________” chunks b) Balance _____ as though it were one item as long as the ion stays together as a group on each side of the yields arrow. 2 3 ___Al + ___CuSO 4 1 2(SO4)3 + ___Al 3 ___Cu start _________ over and begin c) If you can’t seem to get it balanced, _________ with a different element the next time, or put a “2” somewhere and then try again. 2 2 2O ___Li + ___H 2 1 2 ___LiOH + ___H d) This is what I’ll constantly be telling you to do if you are stuck and you need my help... “Pick an element to balance. How many are on Fix it the left side? How many are on the right side? ________ ____!” 2 ___Fe(OH) 3 1 2O3 + ___H 3 2O ___Fe Balancing Equations: “Helpful Hints” e) Mr. Reid’s goofy “balancing song” may help: 2 on the left and a ___on 3 3 on the “If there’s a ___ the right, you put a ___ left and a ___ 2 on the right, (makin’ money!)” ___Al 4 + ___O 3 2 ___Al 2 2O3 f) If you see only C’s H’s and O’s, balance them in this order: C, H, O. ___C 2 2H2 + ___O 5 2 ___CO 4 2 + ___H 2 2O Six General Types of Reactions 1) ________________________: Decomposition • one ______________ compound into simpler A reaction that breaks apart ______ substances, (usually two elements or an element and a smaller compound.) + General Form: _____ AX ___ A + ___ X H2 + _____ O2 Examples: H O _____ 2 K + _____ Cl2 KCl _____ Remember that “HONClBrIF” elements are diatomic when alone!! General Types of Reactions (Continued) 2) _______________: Composition (sometimes called “Combination” or “Synthesis”) • • two __________________, substances A reaction of _____ typically a metal and a nonmetal to form ______ one ______________. compound It is the opposite of decomposition. + General Form: Examples: A + ___ X _____ AX ___ Al + AlCl3 Cl2 _______ K2S K + S ___ General Types of Reactions (Continued) 3) _____________ Single Replacement: • one ______________ compound one ____________ element A reaction between ____ and ___ that produces a different _____________ compound and ______________. element General Forms: ____ AX + __ Y ____ AY + + __ X AX B ____ BX + __ A ____ + __ + • • • • more The element that is trying to replace the other must be ________ reactive _______________ than the one it is replacing. You must use the Activity Series to see if the reaction will happen. Activity Series _________ ___ = more reactive Higher up Elements from ____ Li to ____ Na can displace hydrogen in water to form a metallic hydroxide and H2 gas. Activity Series Single Replacement Reactions Examples: NaCl NaF + _____ Cl2 + F2 _____ FeCl2 + KCl + _____ Fe K _____ HCl + H2 Zn ZnCl _____ 2 + _____ HCl + No Reaction Au _____ + _____ NaOH + _____ H2 H2O + Na _____ H(OH) Ag AgNO3 + Cu CuNO _____3 + _____ General Types of Reactions (Continued) 4) _______________ Double Replacement: (sometimes called “Ionic”) • • two ________________ compounds A reaction between _____ that are dissolved in water that produces _____ two ________________ compounds , one of which is ________________. insoluble Water or a gas may be one of the two compounds being produced. BX(s) (aq)+ BY (aq) AY (aq) + ____ General Form: AX ____ ____ ____ + • • + You must use the Solubility Chart to see which product is the precipitate. I or _____= sS Solubility Chart ___ precipitate Examples: CaCl2 (aq) + AgCl(s) AgNO3 (aq) Ca(NO _________ 3)2 (aq) + ________ NaCl(aq) + ________ H2O (l) NaOH (aq) + HCl (aq) ________ General Types of Reactions (Continued) 5) _________________: Combustion • • • A reaction between a Carbon/Hydrogen (and sometimes Oxygen) O2 _________________ compound with _____. CO2 + ________ H2O The products are always the same… ________ This reaction is too easy!! Don’t miss it! General Form: Examples: C2H2 CO2 + ____ H2O CxHy + O2 ____ + C7H6O + CO2 + _______ H2O O2 _______ CO2 + _______ H2O O2 _______ General Types of Reactions (Continued) 6) _________________: Nuclear Fission and Fusion • There are two types of Nuclear reactions, ________________ – Fission reactions involve a heavy nucleus that will split into two or three pieces. – Fusion reactions involve two light nuclei that combine into a heavier one. – New elements are formed! • You will not be asked to predict products! • Examples of nuclear fission reactions: Nuclear Weapons (atom bomb) General Types of Reactions (Continued) • More examples of nuclear fission reactions: Nuclear Medicine /Medical Imaging Nuclear Power Plants General Types of Reactions (Continued) • Examples of nuclear Fusion reactions: Hydrogen on the sun becomes Helium Fusion