* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Diffusion and Osmosis in plant and animal cells
Survey
Document related concepts
Embryonic stem cell wikipedia , lookup
Dictyostelium discoideum wikipedia , lookup
Regeneration in humans wikipedia , lookup
Cellular differentiation wikipedia , lookup
Chimera (genetics) wikipedia , lookup
Neuronal lineage marker wikipedia , lookup
Cell culture wikipedia , lookup
Human genetic resistance to malaria wikipedia , lookup
Hematopoietic stem cell wikipedia , lookup
Human embryogenesis wikipedia , lookup
Cell (biology) wikipedia , lookup
Artificial cell wikipedia , lookup
Microbial cooperation wikipedia , lookup
State switching wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Transcript
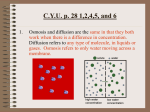
Intermediate 2 Biology Unit 1: Living Cells DIFFUSION AND OSMOSIS IN PLANT AND ANIMAL CELLS Diffusion Learning Objectives • Name the method by which substances move in and out of cells. • Define the term ‘diffusion’ • State when diffusion will stop. • Identify concentration differences and predict the direction of movement of substances by diffusion in a given diagram. • State which substances enter and leave cells by diffusion. • Explain the importance of diffusion to cells. Diffusion • Diffusion is the net movement of molecules of a substance from a region of high concentration to an region of low concentration until the concentrations become equal. • The difference in concentration is called the concentration gradient. Diffusion in Organisms Unicellular organisms • Oxygen diffuses into the cell • Carbon dioxide diffuses out of the cell • Diffusion of food substances into the cytoplasm • Examples – Paramecium – Ameoba Multicellular organisms • Diffusion in the alveoli – O2 into the blood – CO2 into the alveoli • Diffusion between the blood and body cells – O2 from the blood into the body cells – CO2 from the body cells into the blood Role of the cell membrane • The cell surface membrane is selectively permeable – Small molecules can pass freely e.g. Oxygen, water, carbon dioxide – Some molecules e.g. Glucose can pass through more slowly – Large insoluble molecules e.g. Starch can not pass through Osmosis Learning Objectives • Name the method by which water passes into and out of cells. • Explain what a selectively permeable membrane is. • Explain what is meant by a concentration gradient. • Define osmosis using the terms selectively permeable membrane and concentration gradient. • Identify water concentration gradients when given percentage solute concentrations. • Predict the direction of water movement between 2 solutions of known concentration of solute • Describe a visking tubing experiment involving 2 solutions of different concentration and predict its results Osmosis • Osmosis is the net movement of water from a region of high water concentration to a region of low water concentration (down a water concentration gradient) across a selectively permeable membrane. Relative water concentrations • Hypotonic – Solution with the higher water concentration • Isotonic – Solutions of equal water concentrations • Hypertonic – Solution with the lower water concentration Osmosis in plant and animal cells Learning Objectives • Explain why osmotic effects are different on plant cells compared with animal cells. • State the meaning of the terms: hypertonic, hypotonic and isotonic. • Describe the effects on plant cells of immersion in different solutions. • State the meanings of the words: flaccid, turgid and plasmolysed. • Predict the effect of different solutions on plant cells. • Recognise and label plant cells in various conditions. • Describe the effects on animal cells of immersion in different solutions. • Predict the effect of different solutions on animal cells. Osmosis in red blood cells (pg 33 Int 2 Bio 1st Edition) • If a red blood cell is placed in a hypotonic solution it will burst • If a red blood cell is placed in an isotonic solution there is no net movement of water and the cell remains unchanged • If a red blood cell is placed in a hypertonic solution is will shrink (crenate) Plant cells (pg 33 Int 2 Bio 1st Edition) • If a plant cell is placed in a hypotonic solution it becomes turgid • If a plant cell is placed in an isotonic solution there is no net movement of water and the cell remains unchanged • If a plant cell is placed in a hypertonic solution it becomes plasmolysed Osmoregulation in Paramecium • Paramecium lives in fresh water, as a result the organism is continuously gaining water by osmosis • The cell is prevented from bursting by the presence of contractile vacuoles • The contractile vacuoles have canals that collect excess water, when swollen the vacuole contracts and discharges it contents through a pore • There are two contractile vacuoles and they discharge their contents alternately